The formation of carbon-carbon bonds is essential for the construction of the molecular framework of organic molecules through synthesis. A fundamental process for this bond formation is the reaction between a nucleophilic carbon and an electrophilic carbon.
From a mechanistic point of view, these reactions are usually SN2 reactions, where the carbon nucleophile displaces a halide or other leaving group with inversion of the configuration in the alkyl group. Efficient carbon-carbon bond formation requires that SN2 alkylation be the major reaction. We need to consider the following factors: (a) the conditions for generating the carbon nucleophile; (b) the effect of reaction conditions on the structure and reactivity of the nucleophile; and (c) the regio- and stereoselectivity of the alkylation reaction. The reaction can be used with various carbonyl compounds, such as ketones, esters, and amides.
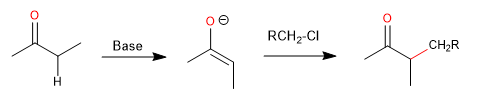
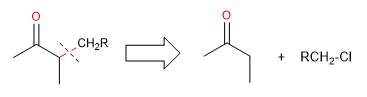
These reactions introduce a new substituent to the carbon adjacent to the carbonyl group and constitute an important method for this transformation. In a retrosynthetic sense, the disconnection is made between the adjacent carbon and a potential alkylating agent.
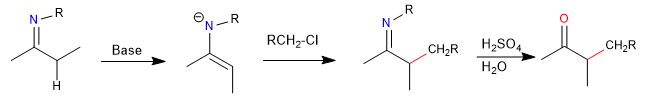
There are similar reactions with nitrogen analogues called imine anions. Alkylated imines can be hydrolyzed to give the corresponding ketone.
Both enolates and imine anions can be used to introduce alkyl substituents into the carbonyl. Since the reaction involves a nucleophilic substitution, primary groups are the best alkylating agents, with methyl, allylic, and benzylic compounds being especially reactive. Secondary groups are less reactive and are likely to give lower yields due to competition with elimination. Tertiary and aryl groups cannot be introduced by an SN2 mechanism.