Acid derivatives behave like bases through carbonyl oxygen. The basicity of this oxygen depends on the resonance stabilization of the conjugate acid.
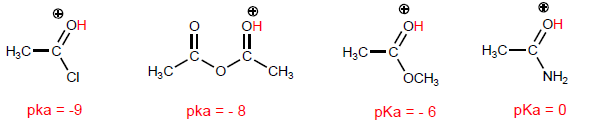
Let's compare the structures that stabilize the base on the halide and the amide.
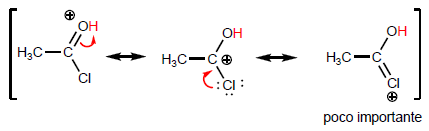
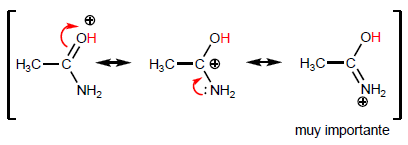
As the last structure gains weight, the acid becomes more stable (weak) and therefore the base stronger. Amides are the strongest bases of all acid derivatives.
Acid derivatives feature acidic hydrogens on carbon.
Alkanoyl halides have the most acidic hydrogens in the a position, while amides have the least acidic.
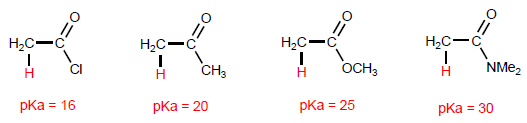
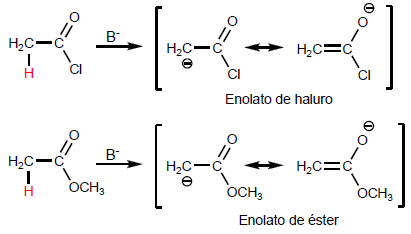
Deprotonation of position produces enolates
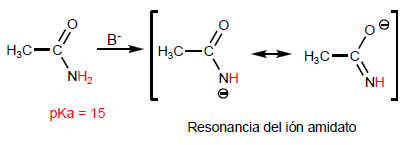
Amides have very acid hydrogens on the nitrogen atom whose subtraction forms amidates.