PYRIDINE THEORY
- Details
- Germán Fernández
- PYRIDINE THEORY
- Hits: 13903
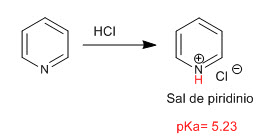
Pyridine behaves as a base through the lone pair of the nitrogen atom. In the presence of acids, it is protonated, generating pyridinium salts.
- Details
- Germán Fernández
- PYRIDINE THEORY
- Hits: 11723
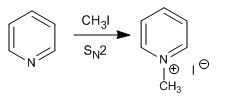
a) Alkylation reaction
Pyridine reacts with alkyl halides, alkanoyl halides, and anhydrides to form pyridinium salts. The reaction proceeds through the nucleophilic attack of the lone pair of nitrogen on the electrophilic carbon of said reagents.
- Details
- Germán Fernández
- PYRIDINE THEORY
- Hits: 10088
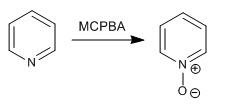
Pyridine is oxidized in the presence of hydrogen peroxide or peracids to form pyridine N-oxides.
- Details
- Germán Fernández
- PYRIDINE THEORY
- Hits: 22769
The pyridine ring is capable of attacking electrophiles, analogously to benzene, giving the aromatic electrophilic substitution reaction. Due to the electronegativity of nitrogen, pyridine is much less reactive than benzene in SE, requiring more drastic reaction conditions.
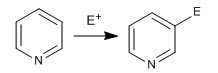
- Details
- Germán Fernández
- PYRIDINE THEORY
- Hits: 1807
It is possible to place electrophiles in position 4 of the pyridine through the formation of N-oxides.
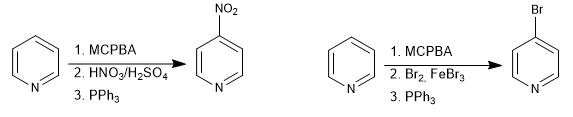
Read more: Electrophilic substitution at position 4 of pyridine
- Details
- Germán Fernández
- PYRIDINE THEORY
- Hits: 1177
This reaction forms organolithiums from a halogenated pyridine. Organolithics allow the attack of very varied carbon electrophiles, such as: primary alkyl halides, aldehydes, ketones, nitriles, esters, epoxides...
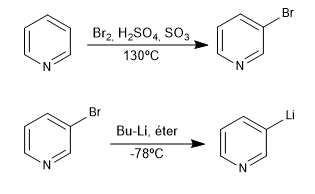
- Details
- Germán Fernández
- PYRIDINE THEORY
- Hits: 1368
Hard nucleophiles (organometallic, amide, lithium aluminum hydride) attack position 2 of the pyridine ring, giving rise to nucleophilic addition reactions. In the event that a final oxidation stage occurs, with loss of hydride, we can speak of nucleophilic substitution.
a) Addition of organometallics
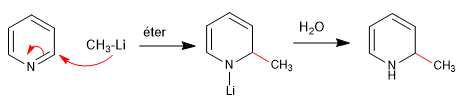
Rearomatization of the ring is possible by adding an oxidant that removes "H 2 "
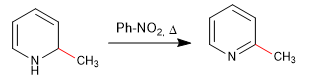
b) Addition of amide. Chichibabin's reaction
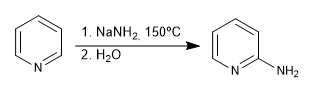
- Details
- Germán Fernández
- PYRIDINE THEORY
- Hits: 6062
Pyridines with leaving groups at positions 2,4 react with nucleophiles, resulting in substitution of the leaving group for the nucleophile. The reaction follows an addition-elimination mechanism.
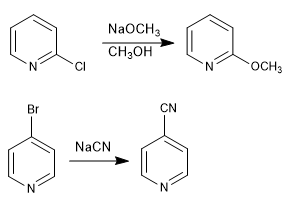
- Details
- Germán Fernández
- PYRIDINE THEORY
- Hits: 1587
Pyridines with leaving groups in position 3 do not give nucleophilic substitutions, but can undergo elimination in the presence of strong bases, leading to an intermediate called pyridine (equivalent to benzyne), which is attacked by the nucleophile in the middle.
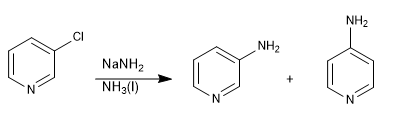
Read more: Nucleophilic substitution by elimination-addition: the pyridine
- Details
- Germán Fernández
- PYRIDINE THEORY
- Hits: 1019
Pyridines with 2,4-alkyl groups have acidic hydrogens in the neighboring position of the ring. These hydrogens can be removed by using strong bases such as tert-butyllithium, LDA, etc.
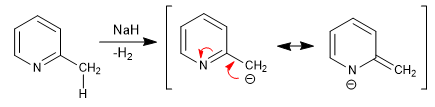
The base obtained has an important nucleophilic character, which allows the chain to be lengthened by attacking different electrophiles.
- Details
- Germán Fernández
- PYRIDINE THEORY
- Hits: 16555
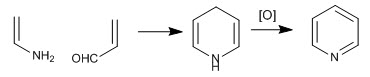
 The pyridine molecule can be obtained by reacting enamines with a,b-unsaturated. Thus, the reaction between ethenamine and propenal, followed by oxidation, produces pyridine.
The pyridine molecule can be obtained by reacting enamines with a,b-unsaturated. Thus, the reaction between ethenamine and propenal, followed by oxidation, produces pyridine.
- Details
- Germán Fernández
- PYRIDINE THEORY
- Hits: 8411
The condensation of two molecules of ethanal and one of methane produces a 1,5-dicarbonyl compound that reacts with ammonia to generate pyridine.
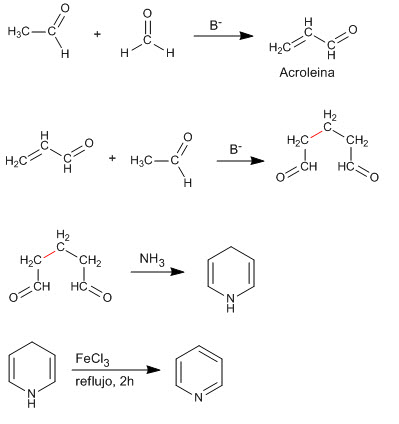
- Details
- Germán Fernández
- PYRIDINE THEORY
- Hits: 1076
The Kröhnke synthesis of pyridine starts from a pyridinium ylide and an alpha, beta-unsaturated to yield 1,5-dicarbonyl, which by reaction with ammonia gives the final pyridine.
- Details
- Germán Fernández
- PYRIDINE THEORY
- Hits: 1978
The Guareschi Thorpe synthesis prepares 2-pyridones from cyanoacetamide as the nitrogenous component, together with a 1,3-diketone or 3-ketoester.









