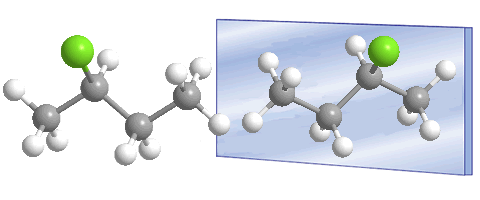
 Compounds with an asymmetric carbon, such as 2-chlorobutane, can exist as two isomers.
Compounds with an asymmetric carbon, such as 2-chlorobutane, can exist as two isomers.
Carbon 2 is asymmetric, it is attached to four different substituents, which are: chlorine, methyl, ethyl and hydrogen. The presence of the asymmetric carbon (chiral center) allows the existence of two stereoisomers (enantiomers) that are differentiated by the different spatial arrangement of the substituents around the asymmetric carbon.
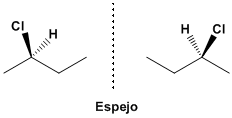
The molecule that results from putting the chlorine towards us is not the same as the molecule that chlorine has in the background. These molecules cannot be superimposed by twists, they are different. Placed properly it can be seen that they are mirror images.