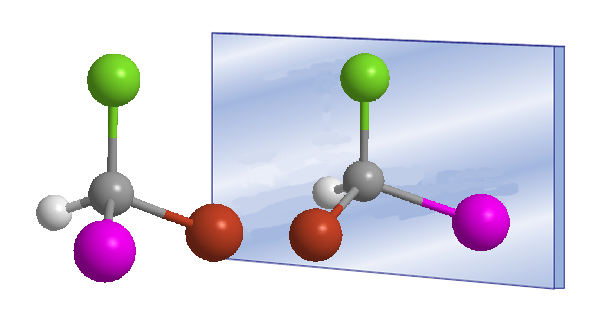
The word chiral was introduced by William Thomson (Lord Kelvin) in 1894 to designate objects that are not superimposable with their mirror image. Applied to organic chemistry, we can say that a molecule is chiral when it and its mirror image are not superimposable.
Chirality is often associated with the presence of asymmetric carbons. An asymmetric carbon is one that is attached to four different substituents. An example of an asymmetric carbon is found in the Bromochloroiodomethane molecule. The carbon is attached to bromine, chlorine, iodine, and hydrogen, four different substituents that make it chiral, or asymmetric. The molecule and its mirror image are different, no twist allows them to be superimposed. The relationship between a molecule and its non-superimposable mirror image is one of enantiomers.
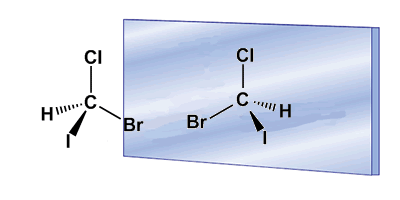
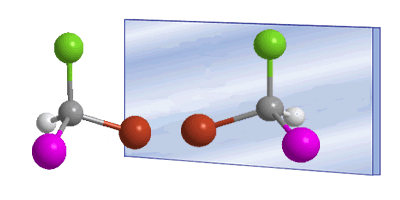
In these drawings we can see the Bromochloroiodomethane molecule and its enantiomer reflected in the mirror.