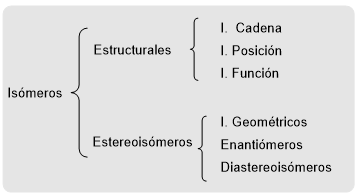
Isomers are molecules that have the same molecular formula but different structures. It is classified as structural isomers and stereoisomers. Structural isomers differ in the way their atoms are bonded and are classified into chain, position, and function isomers. As an example, let's draw the structural isomers of formula C 2 H 6 O .

[1] Ethanol
[2] Dimethyl ether
There are only two ways to unite the atoms that generate different compounds. In ethanol, oxygen is bonded to a carbon and a hydrogen. In dimethyl ether it is attached to two carbons. These are structural isomers since the atoms are bonded differently in both molecules. As they belong to different functional groups (alcohol and ether) they are classified as functional isomers.
Pentane and 2-Methylbutane are single chain isomers, both with the formula C 5 H 12 . Pentane is a straight chain alkane while 2-Methylbutane has a branch.
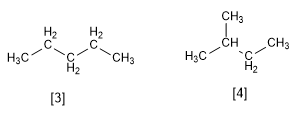
[3] Pentane
[4] 2-Methylbutane
Again note how the atoms are bonded differently in both molecules.
2-Pentanol and 3-Pentanol are positional isomers. The hydroxyl group occupies a different position in each molecule. 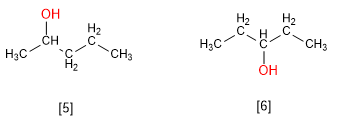
[5] 2-Pentanol
[6] 3-Pentanol
In stereoisomers, the atoms are connected in the same way in both molecules. The difference lies in the different spatial orientation of the atoms or groups of atoms. Stereoisomers are classified as geometric (cis-trans) isomers, enantiomers, and diastereoisomers.