Chirality is synonymous with asymmetry, chiral objects are characterized by the absence of symmetry, look at the hands.
The elements of asymmetry that lead to chiral molecules are: chiral centers, chirality axes, chirality planes, and helices.
a) Chiral or stereogenic center: it is an atom that unites four different groups, one of these four groups can be a lone pair.
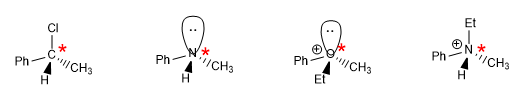
Not only carbon can be the stereogenic center, but also nitrogen from amines or ammonium salts, oxygen in oxonium cations, phosphorus in phosphines......
b) Allenes are chiral molecules, despite not having a stereogenic center, due to the presence of a chirality axis .
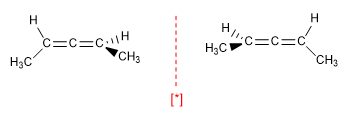
[*] Pair of enantiomers
The axis of chirality requires different groups attached to each carbon and that the groups on one side are perpendicular to those on the other side.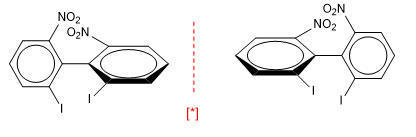
Biphenyls also have axes of chirality.

[*] Pair of enantiomers
c) Planes of chirality. They arise in planar molecules such as benzenes, when a chain connects two ring positions either at the top or bottom. Furthermore, benzene needs some substituent to break the symmetry.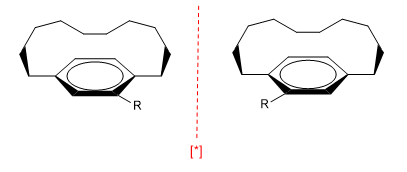
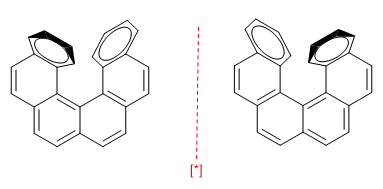
The chain cannot be very long, otherwise the enantiomers interconvert.

[*] Pair of enantiomers
d) Propellers. Helical molecules can also exhibit chirality.

[*] Pair of enantiomers
In this case, the chirality is due to the repulsion of the two upper rings, which forces one of them to twist towards us while the other goes to the bottom.









