STEREOCHEMISTRY II
- Details
- Germán Fernández
- STEREOCHEMISTRY II
- Hits: 1835
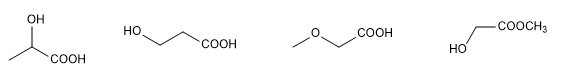
The determination of the structure of an organic molecule begins with the analysis of the elements it contains and their proportion, which is usually done by combustion. Molecular mass determination, previously performed by cryoscopic descent, now uses the high-resolution mass spectrometry technique.
- Details
- Germán Fernández
- STEREOCHEMISTRY II
- Hits: 2107
The different spatial arrangements that a molecule can adopt and that are interconverted at room temperature by rotation are called conformations.
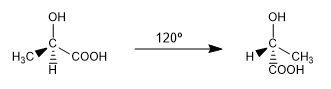
They are two of the infinite conformations that can be drawn from the ac. 2-hydroxypropanoic. At room temperature the molecule is continuously rotating through all possible conformations.
Now let's look at the two most characteristic conformations of ethane, the alternate and eclipsed conformations.
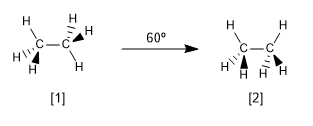
Alternate conformation of ethane
Eclipsed conformation of ethane
- Details
- Germán Fernández
- STEREOCHEMISTRY II
- Hits: 2265
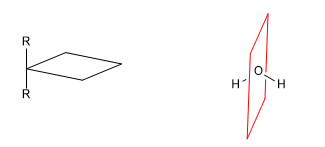
A chiral molecule is one that is not superimposable with its mirror image. Symmetry causes molecules to lose their chirality. Thus, the presence of planes of symmetry, inversion centers or alternating axes give rise to achiral molecules.
a) Axis of symmetry (C n )
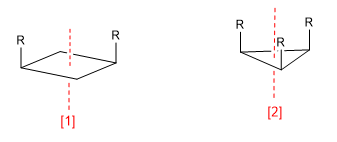
Symmetry axis (C 2 )
Symmetry axis (C 3 )
An axis of symmetry of order m leaves the molecule in a configuration indistinguishable from the initial one when rotating 360/m degrees.
b) Plane of reflection ( s ): Divides the molecule into two equal parts. Every atom of the molecule that is on one side of the plane must have its mirror on the other side.
Read more: Elements of symmetry leading to achiral molecules
- Details
- Germán Fernández
- STEREOCHEMISTRY II
- Hits: 2164
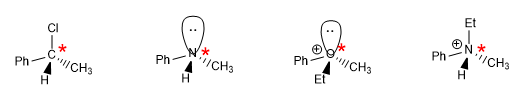
Chirality is synonymous with asymmetry, chiral objects are characterized by the absence of symmetry, look at the hands.
Read more: Elements of asymmetry leading to chiral molecules
- Details
- Germán Fernández
- STEREOCHEMISTRY II
- Hits: 1660
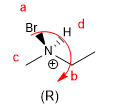
The rules to give absolute configuration to a stereogenic center are the following:
- Details
- Germán Fernández
- STEREOCHEMISTRY II
- Hits: 2283
In molecules whose chirality element is an axis, we will give the notation R a / S a , (the subscript "a" refers to axial), by means of the Fischer projection method. The tetrahedron method can also be used.
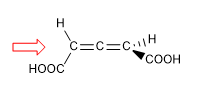
1. For the molecule to have a chirality axis, it is necessary that the two groups on each side are different from each other. In this example the groups are different (-H and -COOH) and the molecule has a chirality axis.
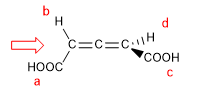
- Details
- Germán Fernández
- STEREOCHEMISTRY II
- Hits: 2422
These are molecules that have a flat area (phenyl), with a bridge that joins their ends either on the top or bottom face.
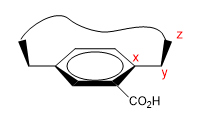
1. We assign names to certain atoms as shown in the image. "z" is the first atom that is out of plane. Two chains start from x, to which we must give priority by atomic number.
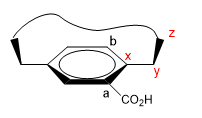
2. We project the xy link, placing ourselves in the position of the arrow.
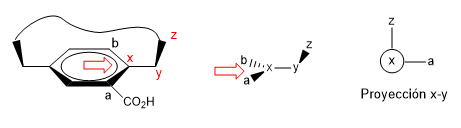
- Details
- Germán Fernández
- STEREOCHEMISTRY II
- Hits: 2154
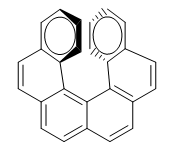
The repulsion between the rings prevents the molecule from being arranged in the plane. So one of the rings bends towards us and the other at the bottom. In one enantiomer the ring on the right is bent towards us and in the other to the bottom.
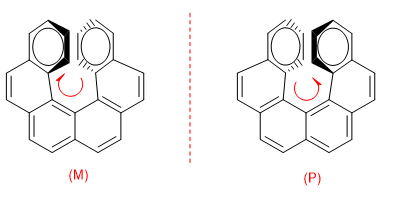
To distinguish both enantiomers, a turn is made from the ring that goes to the bottom to the one that comes towards us. If this turn is clockwise, the enantiomer is M, if the turn is in the opposite direction, the enantiomer is P.









