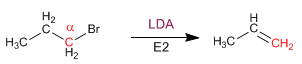ELIMINATION REACTIONS
- Details
- Germán Fernández
- ELIMINATION REACTIONS
- Hits: 61562
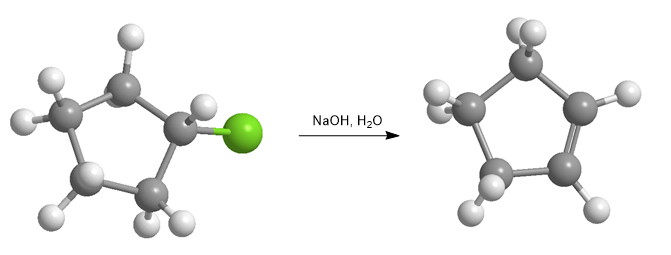
Haloalkanes react with strong bases to form alkenes.
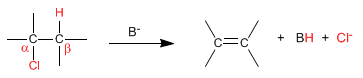
- Details
- Germán Fernández
- ELIMINATION REACTIONS
- Hits: 42846
Bimolecular (E2) eliminations take place at a faster rate if the leaving group is on the opposite side of the removed hydrogen. This spatial arrangement is known as "ANTI"
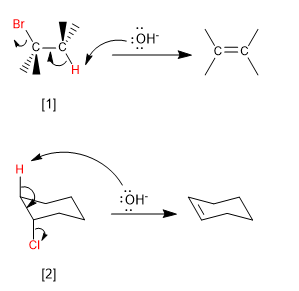
[1] H and Br in "ANTI"
[2] H and Cl in "ANTI"
- Details
- Germán Fernández
- ELIMINATION REACTIONS
- Hits: 72313
Unimolecular deletions (E1) compete with unimolecular nucleophilic substitution (SN1), forming alkenes.
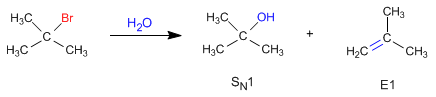
The nucleophilic behavior of water generates the substitution product (S N 1), while the basic character gives rise to the elimination product (E1).
The mechanism of the E1 reaction occurs with the following steps:
- Details
- Germán Fernández
- ELIMINATION REACTIONS
- Hits: 67026
Primary Substrates: Primary substrates have a single carbon chain on the alpha carbon. The outgoing group is not taken into account.
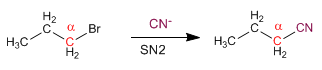
Primary substrates give SN2 with good nucleophiles, such as: I- , Cl- , Br- , NH3 , N3- , CN- , HS- , CH3S- , OH- , CH3O- , NH2- .
Primary substrates do not react with bad nucleophiles: water, alcohols, and acetic acid.
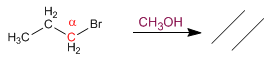
Primary substrates give E2 with hindered bases: potassium tert-butoxide and LDA