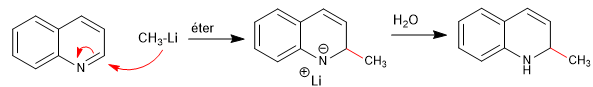
The nucleophiles add to carbon 2 of the quinoline, although under certain conditions it can also receive attacks on its carbon 4.
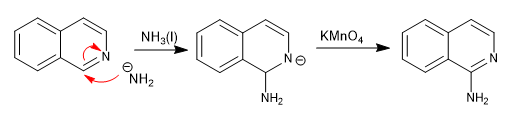
Isoquinoline is attacked by hard nucleophiles on carbon 1.
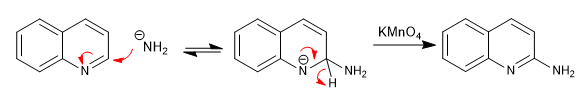
For this reaction to take place, the delocalization of the negative charge on the ring nitrogen is essential.
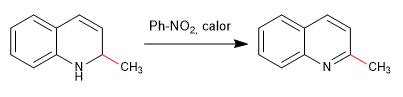
Addition of an oxidant allows rearomatization of the priridine ring
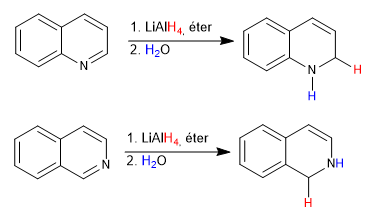
Lithium aluminum hydride contributes hydrides to carbon 2 of quinoline and carbon 1 of isoquinoline
The amide ion, dissolved in liquid ammonia, adds under kinetic conditions to carbon 2 of the quinoline, while under thermodynamic conditions it adds to carbon 4
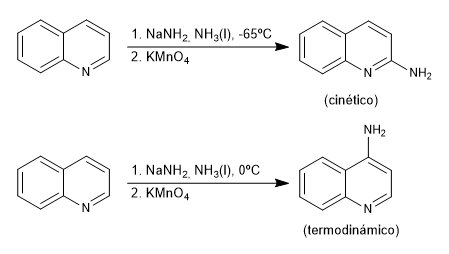
Mechanism:
Isoquinoline undergoes nucleophilic additions at position 1.