a) Leaving group near the ring
The ring expels the leaving groups that are in a neighboring position, this position being attacked by the nucleophiles in the middle.
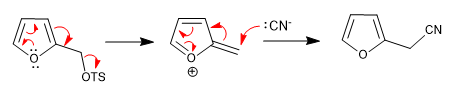
The reaction takes place whether the chain is in position 2 or position 3.
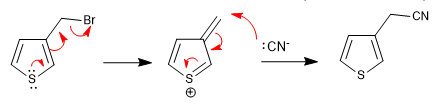
b) Carboxylic acids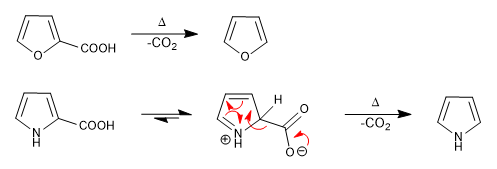
Carboxylic groups in position 2 or 3 of the ring decarboxylate on heating

c) Hydroxy derivatives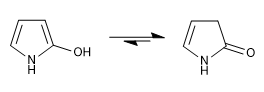
The keto form is more stable than the enol form in both positions 2 and 3.

d) Carbonyls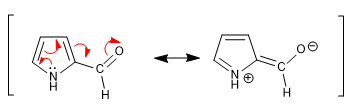
The carbonyl group attached to the pyrrole ring is not very reactive towards nucleophiles due to the transfer of charge by the ring.










