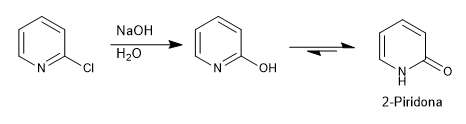
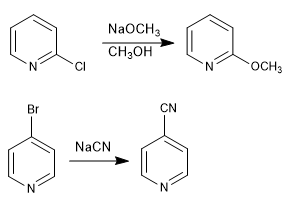
Pyridines with leaving groups at positions 2,4 react with nucleophiles, resulting in substitution of the leaving group for the nucleophile. The reaction follows an addition-elimination mechanism.
Mechanism:
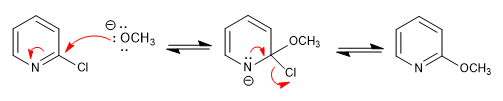
Nucleophilic additions are very slow in position 3 given the impossibility of carrying the charge to the heteroatom.
In the case of adding hydroxide ions to the pyridine, the final tautomerism must be taken into account.