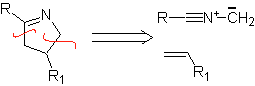
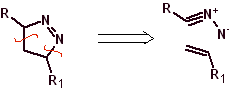
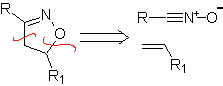
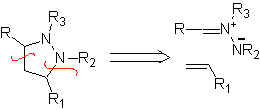
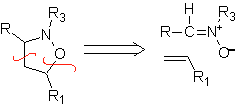
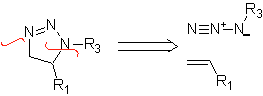
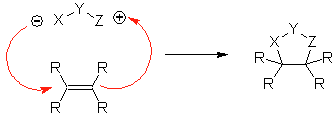
These reactions normally form five-membered heterocyclic rings, for which the reaction between a dipolar n1,3 compound and an alkene is necessary. The reaction is a [3-2] cycloaddition. The 1,3-dipolar compounds that have had the most use to form pentagonal heterocycles are:
 |  |
 |  |
 |  |
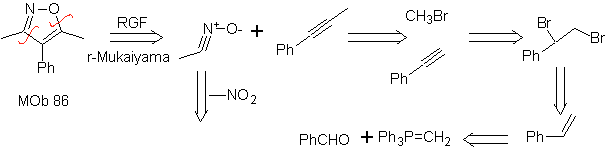
| MOb: 86
| . | MOb: 87 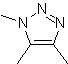 | .. | MOb: 88 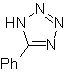 |
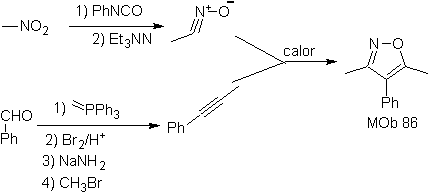
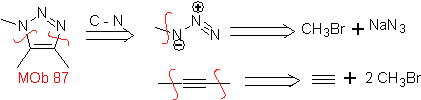
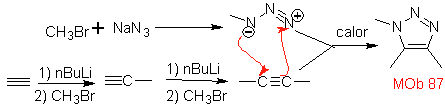
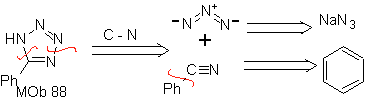
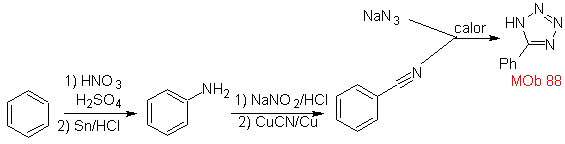
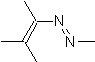
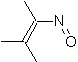
They are good heterodienes, aldehydes and conjugated ketones, 1-azadienes and 2-azadienes. Conjugated aldehydes and ketones produce pyranic rings, the reaction is facilitated by Lewis acids or increased pressure, as well as the presence of an electron group. attractive to the alkene.
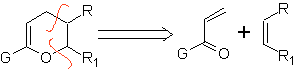
Most common heterodienophiles in the Diels-Alder reaction:
| | ||||||
Aldehydes, ketones and aldehydes, if they are deficient in electrons, react under mild conditions, otherwise they require high pressure and temperature conditions or Lewis acid type catalysts. These heterodienophiles allow the formation of pyranic systems, useful in the preparation of acyclic precursors of various drugs, as is the case of macrocyclic antibiotics.
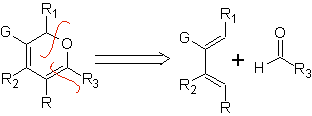 | … | 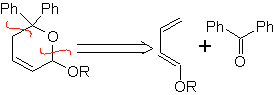 |
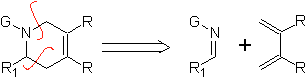 | 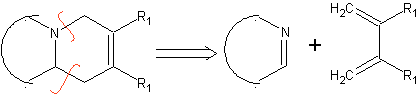 |
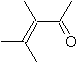 | 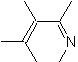 | |
| ||
 |  | |
|
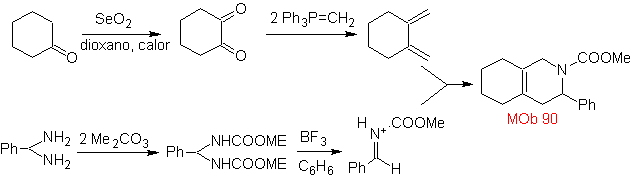
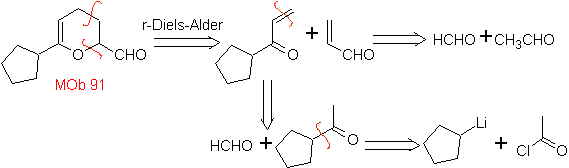
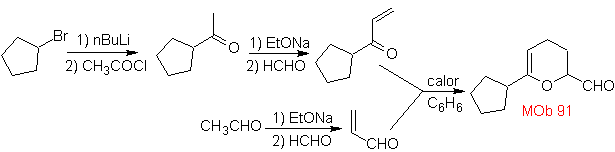
MOb 89 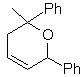 | … | MOb: 90 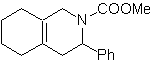 | . | MOb: 91 |
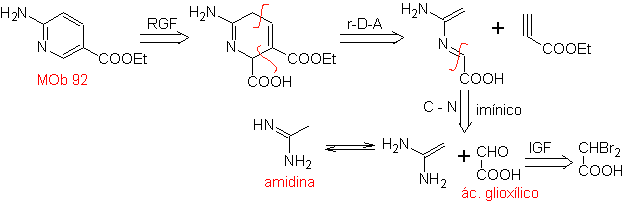
| MOb: 92
| MOb: 93
| MOb 94 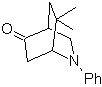 |
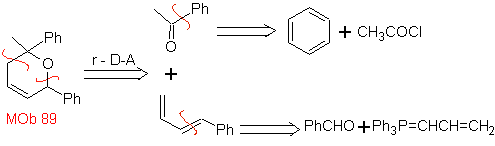
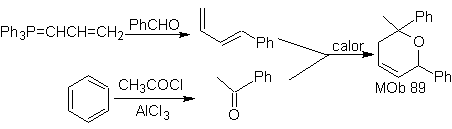
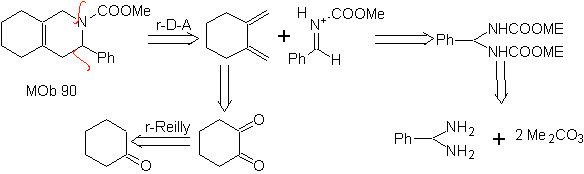
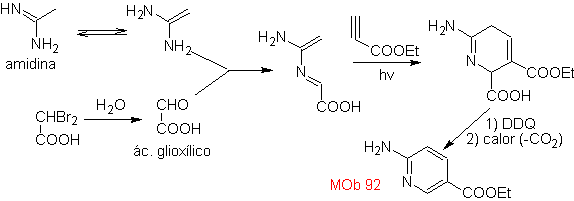
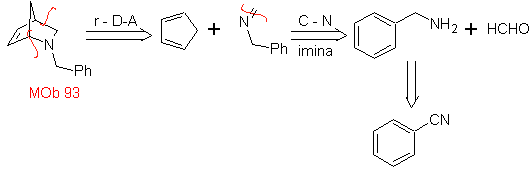
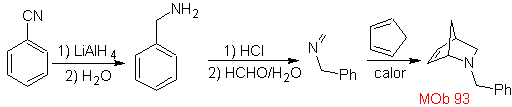
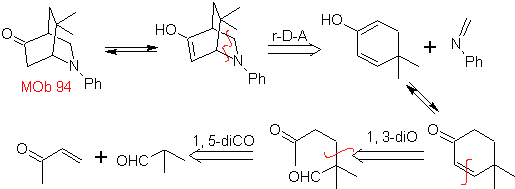
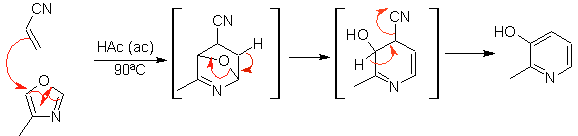
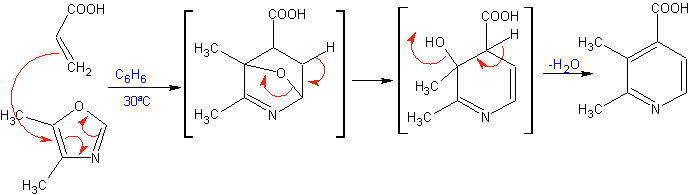
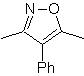
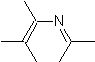
The most studied cycloadditions are those that occur between pentagonal heterocyclic compounds such as oxazoles and some dienophiles, which can be combined with them by electrocyclic addition and subsequent elimination of a small molecule, to produce pyridine rings. Examples: