DISCONNECTION OF 1,4-DIOXYGEN COMPOUNDS
Another group of compounds of great importance in chemical synthesis is made up of dioxygenated molecules that are found in a distance ratio of 1.4. These compounds, when subjected to a retrosynthetic disconnection analysis, generate synthons, where one of them, the electrophile or nucleophile, can be considered "anomalous" or "illogical", because the charge assigned to one of the atoms does not can be explained in terms of its intrinsic or induced electronegativity.
1. 1,4-dioxygen compounds (1,4-diO)
In this type of compounds, the disconnection also leads to a logical synthon and to another illogical (non-natural) synthon, which can be a nucleophile or an electrophile, whose synthetic equivalent still has to be adequately reworked, in order to be used in the reaction. chemistry.
1. 1. Compounds 1,4-dicarbonyl
1.1.1. 1,4-diketone compounds
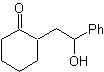
The disconnection alternatives of this type of compounds or molecules to be synthesized (MOb), can lead to the following options:
to. A logical anion synthon and an illogical cation synthon
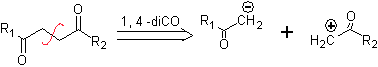
The synthetic equivalent of the anion is the enolate ion or the enol itself of the carbonyl compound. Instead the synthetic equivalent for the carbocation is alpha halocarbonyl. (Umpoloung)
b. A logical cation synthon and an illogical anion synthon
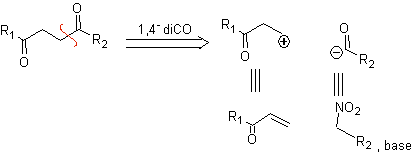
The synthetic equivalent of the logical cation synthon is the α,β–unsaturated carbonyl compound. A suitable synthetic equivalent for the anion synthon may be a nitroalkane anion. The –NO 2 group in alkanes can be transformed into C=O, by means of the Nef reaction, or by the variants of the McMurry reaction, where by action of TiCl 3, the nitroalkane is transformed into an imine, which is then It is hydrolyzed in an acid medium to the respective carbonyl compound.
1.1.2.
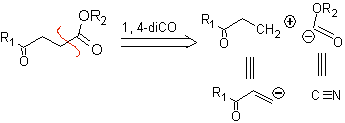
1,4-ketoester compounds
γ-Ketoesters, 1,4-diesters, and 1,4-diacids can be disconnected to a natural cation synthon, the synthetic equivalent of which is an α,β-unsaturated carbonyl compound, and to the non-natural (“illogical”) anion synthon. (-) COOR, whose synthetic equivalent is the cyanide ion.
Examples: Propose a synthesis design, from simple and affordable materials, for each of the following molecules:
MOb 35
| MOb 36
| |
MOb 37
| MOb 38
|
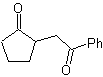
MOb 35 (a). Retrosynthetic analysis . The molecule can be disconnected according to the 1,4-diCO model. The generated precursor cyclopentanone must be previously activated so that its Cα is more nucleophilic, and then be used in the reaction with α.bromoacetone.
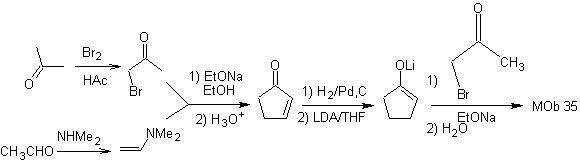
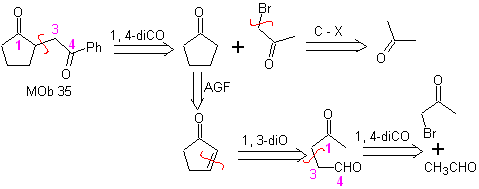 synthesis . The nucleophilicity of cyclopentanone is controlled and guaranteed, using LDA, to arrive at
synthesis . The nucleophilicity of cyclopentanone is controlled and guaranteed, using LDA, to arrive at
MOb 35 (b). Retrosynthetic analysis . The 1,4-diCO model that presents
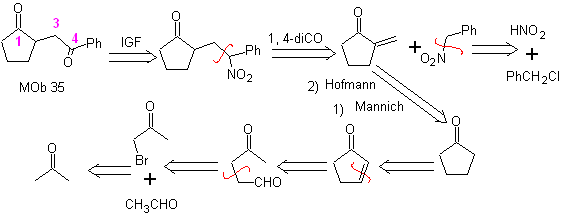
synthesis . Likewise, the last stage, to reach
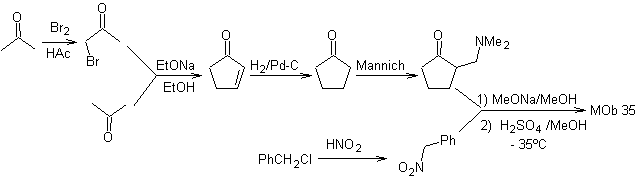
The use of secondary nitroalkanes or nitroarenes generates ketones as a product, by the Nef reaction, which is why this methodology is only applicable to 1,4-ketoesters, 1,4-ketones, and 1,4-ketoaldehydes compounds.
MOb 36. Retrosynthetic analysis. In the first instance, the carboxylic group of
It should be understood that the double bond in the ring is more reactive to epoxidation than the other double bond. The subsequent disconnection α,β-insat CO, allows to form a structure that is easier to disconnect by the models dioxygenates generated in the intermediate molecules.
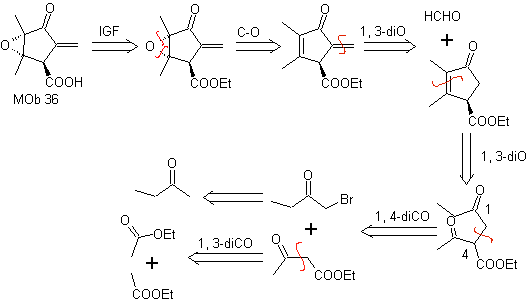
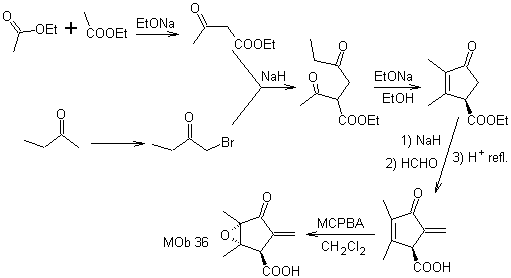
Synthesis. The only care that must be taken in this synthesis is the epoxidation of the ring double bond, more reactive than the vinyl double bond, then the reactions to reach the synthesis of
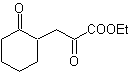
MOb 37. Retrosynthetic analysis: The disconnection of
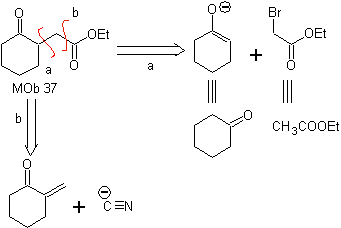
Synthesis : Disconnection (a) follows. hydrolysis
in the end it must be controlled, to
not affect the ester group of MOb 37
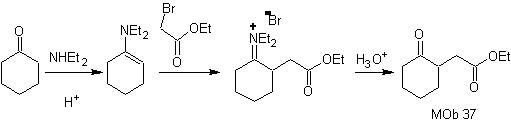
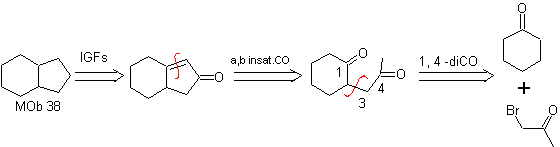
synthesis .
The enamine of cyclohexanone is used again to displace the halogen of the ketone. The 1,4-diCO compound formed is cyclized in a basic medium.
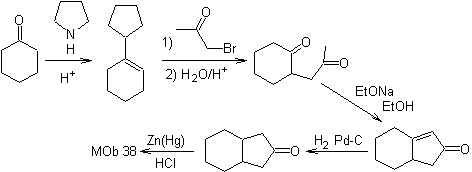
2.1.2.
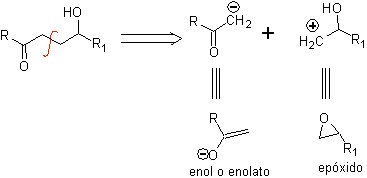
g -Hydroxycarbonyl compounds
A γ-hydroxycarbonyl compound corresponds to the 1,4-diO model, which is why its disconnection provides a logical anionic synthon and an illogical cationic synthon, whose synthetic equivalent can be an epoxide.
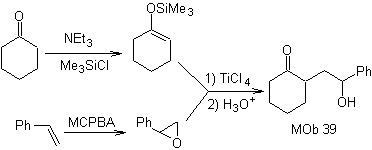
How could the following molecule be synthesized? | MOb 39
|
MOb 39. Retrosynthetic analysis.
The disconnection of the model
γ-hydroxycarbonyl, MOb 39, gives rise to cyclohexanone as a precursor molecule and an aromatic epoxide. You have to look for the enol or enolate of the ketone, which activates its Cα, to open the epoxide on the less hindered side.
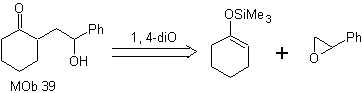
Synthesis. Silylene ether is a powerful nucleophile, capable of attacking an epoxide from the less protected side, in an acid medium and catalyzed by Ti(IV) salt.