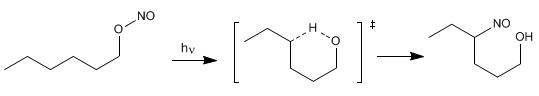
The Barton reaction produces 4-nitrosoalcohols [2] from nitrites [1] by irradiation with ultraviolet light.
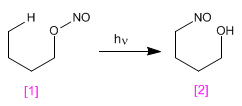
The mechanism is radical and occurs by homolytic cleavage of the O-N bond, forming nitrous oxide and an alkoxide radical. In a later stage, hydrogen subtraction occurs, through a six-membered transition state, which generates a new radical capable of reacting with the nitrous oxide formed in the first step.
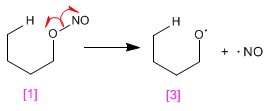
Stage 1. NO link break
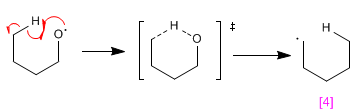
Stage 2. Intramolecular hydrogen subtraction
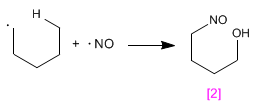
Stage 3. Reaction with radical NO
Examples:
References:
1. DHR Barton, JM Beaton, LE Geller, MM Pechet, J. Am. Chem. Soc. 1960, 82, 2640–2641.
2. DHR Barton, Pure Appl. Chem. 1968, 16, 1–15.