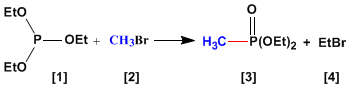REACTIONS IN ORGANIC CHEMISTRY
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 12385
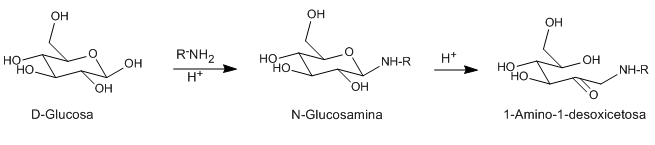
Acids and bases catalyze the isomerization of N-glycosides (glycosamines) from aldoses to 1-aminodeoxyketoses. This rearrangement can be catalyzed with a number of Lewis acids (CuCl 2 , MgCl 2 , AlCl 3 , SnCl 4 ). The amine used as a reagent can be primary or secondary, aliphatic or aromatic.
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 11893
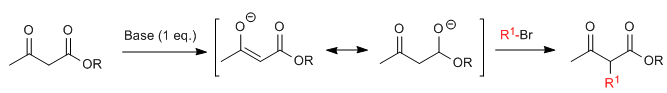
Acetylacetic synthesis allows ketones to be prepared by C-alkylation of ethyl acetylacetate (ethyl 3-oxobutanoate). Ethyl acetoacetate can be deprotonated at the C2 or C4 positions depending on the type and amounts of base used. The hydrogens at the C2 position they present significant acidity (pKa=11) due to stabilization of the conjugate base on the two neighboring carbonyls.In the presence of a base equivalent (alkoxides, LDA, NaHMDS, etc.) a ketoester enolate capable of attacking numerous carbonated electrophiles.
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 15789
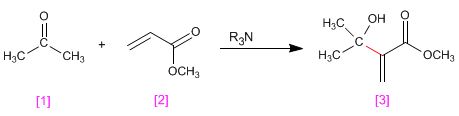
Aldehydes and ketones [1] react with a,b -unsaturated compounds [2] in the presence of tertiary amines that act as catalysts, to form a -hydroxyalkylated products [3] .
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 16026
The Barton reaction produces 4-nitrosoalcohols [2] from nitrites [1] by irradiation with ultraviolet light.
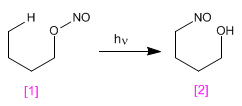
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 22682
The tosylhydrazones of aldehydes or ketones react with two equivalents of organolico to generate an anionic intermediate capable of undergoing alkylation processes.
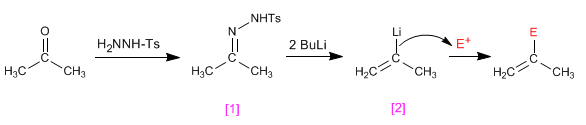
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 18441
The Baeyer Villiger oxidation allows the transformation of ketones into esters.
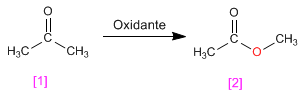
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 19389
The Claisen condensation involves the reaction of esters in basic medium forming 3-ketoesters
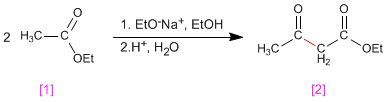
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 26771
acetyl chloride it is treated with diazomethane yielding the diazonium salt . chloride produced reacts with the diazonium salt to give the α-chloroketone . 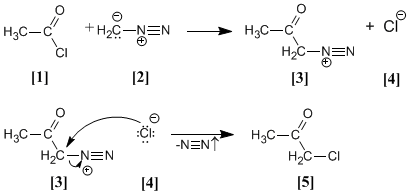
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 17794
Tosylhydrazones of aliphatic aldehydes or ketones react with strong bases to give alkenes . 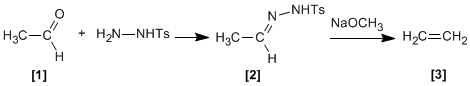
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 32640
In this reaction two alkenes and are treated with a transition metal that acts as a catalyst, giving a mixture of alkenes (including Z/E isomers). This product is obtained by exchange of alkylidene groups. 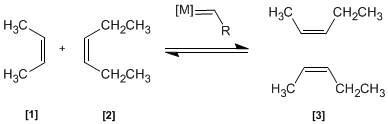
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 28013
Aldol condensation is a reaction of aldehydes or ketones. which forms 3-hydroxycarbonyls (aldols) . 3-hydroxyaldehyde under dehydration conditions by heating yields an alpha, beta-unsaturated aldehyde . 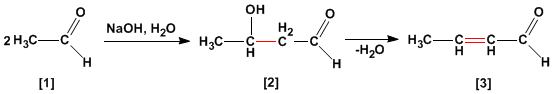
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 24764
Acyloinic condensation transforms esters into alpha-hydroxyketones. This reaction is carried out with sodium metal in an inert solvent. 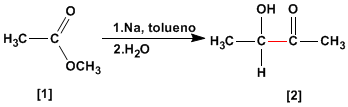
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 22232
The Abuzov reaction is used in the synthesis of phosphonates from phosphites . The phosphonates obtained in the Abuzov synthesis are used as starting materials in the Horner-Wittig synthesis.