REACTIONS IN ORGANIC CHEMISTRY
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 20598
Aldehydes, without alpha hydrogens, give the Cannizzaro reaction on treatment with a strong base (NaOH)
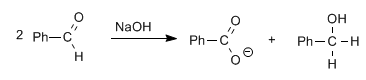
In this reaction one molecule is reduced to alcohol, while the other is oxidized to carboxylic acid.
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 9490
The Blanc reaction allows the chloromethylation of aromatic compounds.
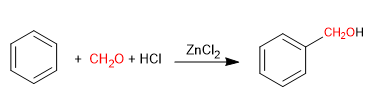
It uses as reagents methane with gaseous hydrogen chloride in the presence of a Lewis acid. The result is the introduction of a hydroxymethyl group on the aromatic ring (benzene) whose hydroxyl is replaced by chlorine in the presence of hydrogen chloride.
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 12677
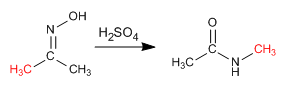
The Beckmann reaction produces the rearrangement of an oxime into an amide. This reaction is carried out in an acid medium.
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 14151
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 9189
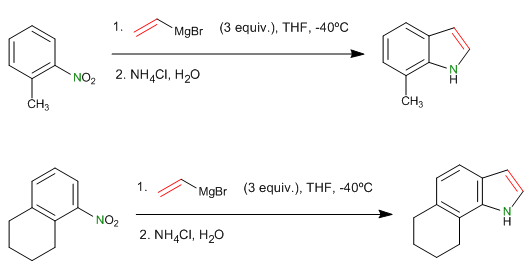
The Barbier reaction makes it possible to obtain unstable organometallic reagents in the reaction medium. Initially it was carried out with Magnesium metal, generating magnesium in situ. Later it was extended to other metals: Sn, Zn..., being able to work in aqueous media, without requiring the protection of acid groups (hydroxyls).
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 10533
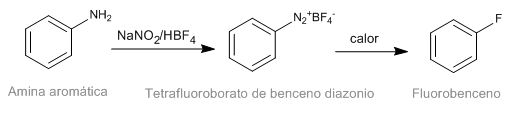
The Schiemann reaction consists of the thermal decomposition of aromatic diazonium tetrafluoroborates to yield the corresponding fluorinated derivative. Although diazonium salts are intestable, diazonium tetrafluoroborates have significant stability and can be prepared in good yield. Diazonium tetrafluoroborate is prepared from aromatic amines by the diazotization reaction.
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 8363
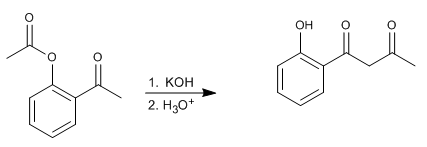
The Baker-Venkataraman rearrangement transforms aromatic ortho-acyloxyketones to beta-diketones by basic treatment (catalysis). Beta-diketones are of great interest in the synthesis of chromones, flavones and coumarins. The most commonly used bases in the reaction are: KOH, potassium tert-butoxide, sodium in toluene, potassium hydride.
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 9941
1,5-Dienes isomerize by sigmatropic-[3,3] rearrangements on heating. Reaction known as Cope rearrangement. The rearrangement of N-substituted 1,5-dienes is called an aza-Cope rearrangement. Depending on the position occupied by nitrogen we have: 1-aza-, 2-aza-, 3-aza-Cope. The 3-aza-Cope regrouping coincides with the aza-Claisen.
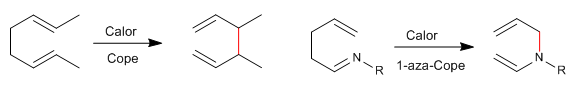
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 9679
Sigmatropic [3,3] rearrangements of N-allyleneamines are known as aza-Claisen rearrangements. This reaction is analogous to the Claisen rearrangement of allyl vinyl ethers.
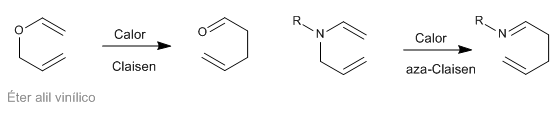
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 8563
Reaction analogous to the Wittig rearrangement, in which alpha metal ethers rearrange alkoxides, generating secondary or tertiary alcohols after hydrolysis. In the case of aza-[2,3]-Wittig, these are alpha metallated allylic tertiary amines which produce secondary amines after hydrolysis.

- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 12376
Acids and bases catalyze the isomerization of N-glycosides (glycosamines) from aldoses to 1-aminodeoxyketoses. This rearrangement can be catalyzed with a number of Lewis acids (CuCl 2 , MgCl 2 , AlCl 3 , SnCl 4 ). The amine used as a reagent can be primary or secondary, aliphatic or aromatic.
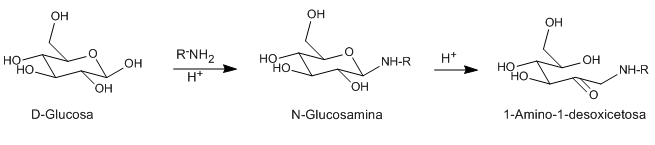
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 11885
Acetylacetic synthesis allows ketones to be prepared by C-alkylation of ethyl acetylacetate (ethyl 3-oxobutanoate). Ethyl acetoacetate can be deprotonated at the C2 or C4 positions depending on the type and amounts of base used. The hydrogens at the C2 position they present significant acidity (pKa=11) due to stabilization of the conjugate base on the two neighboring carbonyls.In the presence of a base equivalent (alkoxides, LDA, NaHMDS, etc.) a ketoester enolate capable of attacking numerous carbonated electrophiles.
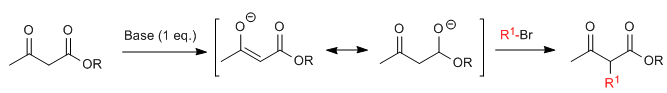
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 15776
Aldehydes and ketones [1] react with a,b -unsaturated compounds [2] in the presence of tertiary amines that act as catalysts, to form a -hydroxyalkylated products [3] .
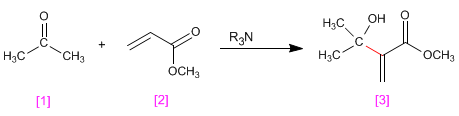
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 16005
The Barton reaction produces 4-nitrosoalcohols [2] from nitrites [1] by irradiation with ultraviolet light.
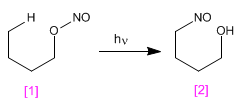
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 22647
The tosylhydrazones of aldehydes or ketones react with two equivalents of organolico to generate an anionic intermediate capable of undergoing alkylation processes.
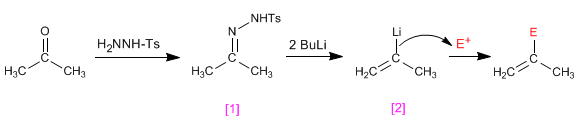
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 18414
The Baeyer Villiger oxidation allows the transformation of ketones into esters.
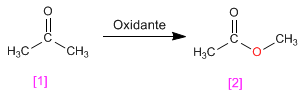
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 19375
The Claisen condensation involves the reaction of esters in basic medium forming 3-ketoesters
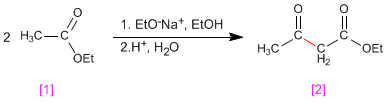
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 26764
acetyl chloride it is treated with diazomethane yielding the diazonium salt . chloride produced reacts with the diazonium salt to give the α-chloroketone . 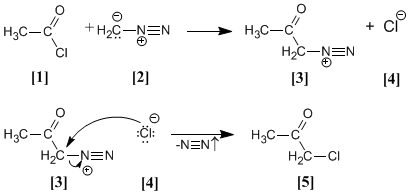
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 17775
Tosylhydrazones of aliphatic aldehydes or ketones react with strong bases to give alkenes . 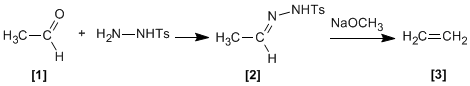
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 32623
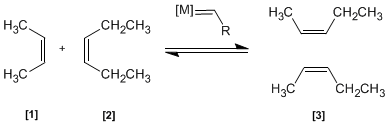
In this reaction two alkenes and are treated with a transition metal that acts as a catalyst, giving a mixture of alkenes (including Z/E isomers). This product is obtained by exchange of alkylidene groups.