REACTIONS IN ORGANIC CHEMISTRY
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 11234
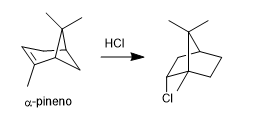
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 12768
The Wacker oxidation allows the transformation of alkenes into the corresponding ketones by treatment with Pd in the presence of copper salts. Copper has the ability to oxidize palladium(0) to palladium(II), the latter being the catalytic agent in the reaction.
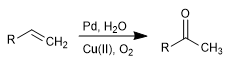
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 13410
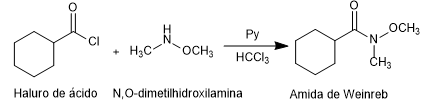
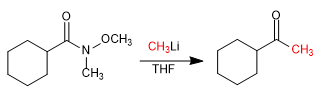
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 7441
In 1902, Wolff observed that treating diazoacetophenone (α-diazoketone) with Ag2O/H2O produced a rearrangement that generated phenylacetic acid.
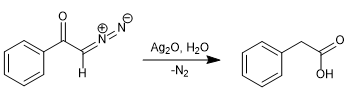
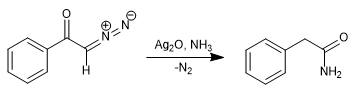
By replacing water with ammonia, phenylacetamide is obtained.
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 14466
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 22716
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 10586
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 8250
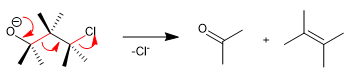
The Wharton fragmentation is a concerted reaction in which a leaving group is located in position 4 with respect to an electron donating group. The transfer of the free pairs of the donor group produces the fragmentation of the neighboring bond and the loss of the leaving group with the formation of double bonds. Let's see an example:
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 7110
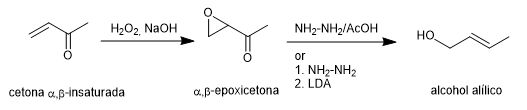
The Wharton synthesis allows α,β-epoxyketones to be transformed into allylic alcohols by treatment with hydrazine in acetic acid medium or hydrazine hydrate followed by strong base. The $\alpha,\beta$-epoxyketone is obtained from the $\alpha,\beta$-unsaturated ketone by oxidation with hydrogen peroxide in a basic medium.
Read more: Wharton synthesis of olefins (Wharton rearrangement)
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 11053
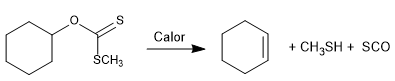
The Chugaev reaction allows the formation of olefins from xanthans.
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 20599
Aldehydes, without alpha hydrogens, give the Cannizzaro reaction on treatment with a strong base (NaOH)
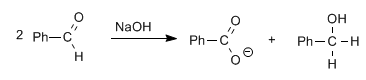
In this reaction one molecule is reduced to alcohol, while the other is oxidized to carboxylic acid.
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 9491
The Blanc reaction allows the chloromethylation of aromatic compounds.
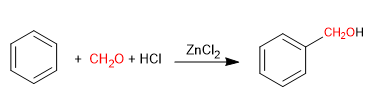
It uses as reagents methane with gaseous hydrogen chloride in the presence of a Lewis acid. The result is the introduction of a hydroxymethyl group on the aromatic ring (benzene) whose hydroxyl is replaced by chlorine in the presence of hydrogen chloride.
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 12677
The Beckmann reaction produces the rearrangement of an oxime into an amide. This reaction is carried out in an acid medium.
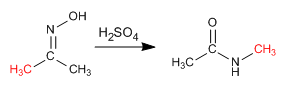
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 14151
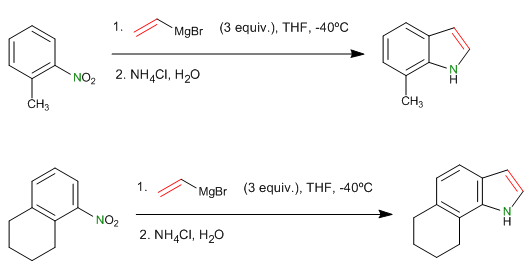
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 9189
The Barbier reaction makes it possible to obtain unstable organometallic reagents in the reaction medium. Initially it was carried out with Magnesium metal, generating magnesium in situ. Later it was extended to other metals: Sn, Zn..., being able to work in aqueous media, without requiring the protection of acid groups (hydroxyls).
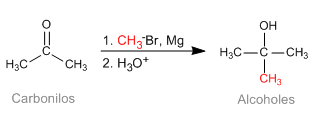
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 10534
The Schiemann reaction consists of the thermal decomposition of aromatic diazonium tetrafluoroborates to yield the corresponding fluorinated derivative. Although diazonium salts are intestable, diazonium tetrafluoroborates have significant stability and can be prepared in good yield. Diazonium tetrafluoroborate is prepared from aromatic amines by the diazotization reaction.
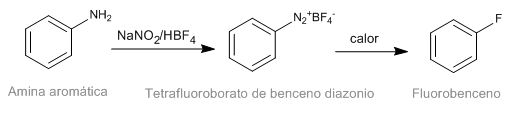
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 8363
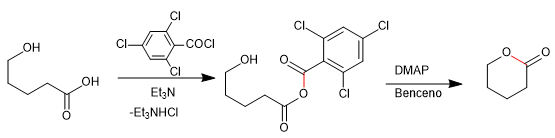
The Baker-Venkataraman rearrangement transforms aromatic ortho-acyloxyketones to beta-diketones by basic treatment (catalysis). Beta-diketones are of great interest in the synthesis of chromones, flavones and coumarins. The most commonly used bases in the reaction are: KOH, potassium tert-butoxide, sodium in toluene, potassium hydride.
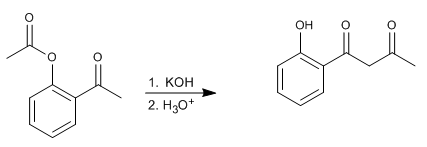
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 9941
1,5-Dienes isomerize by sigmatropic-[3,3] rearrangements on heating. Reaction known as Cope rearrangement. The rearrangement of N-substituted 1,5-dienes is called an aza-Cope rearrangement. Depending on the position occupied by nitrogen we have: 1-aza-, 2-aza-, 3-aza-Cope. The 3-aza-Cope regrouping coincides with the aza-Claisen.
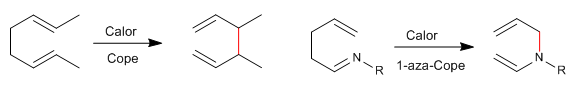
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 9679
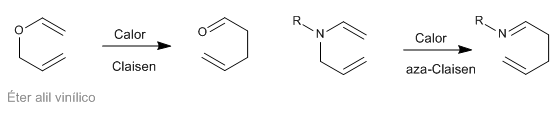
Sigmatropic [3,3] rearrangements of N-allyleneamines are known as aza-Claisen rearrangements. This reaction is analogous to the Claisen rearrangement of allyl vinyl ethers.
- Details
- Germán Fernández
- REACTIONS IN ORGANIC CHEMISTRY
- Hits: 8563
Reaction analogous to the Wittig rearrangement, in which alpha metal ethers rearrange alkoxides, generating secondary or tertiary alcohols after hydrolysis. In the case of aza-[2,3]-Wittig, these are alpha metallated allylic tertiary amines which produce secondary amines after hydrolysis.










