The nitriles are hydrolyzed with aqueous soda, under heating, to form carboxylates and ammonia.
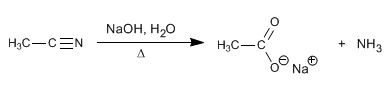
The mechanism of basic hydrolysis takes place in the following steps:
Stage 1 . Nucleophilic attack of the hydroxide ion on the electrophilic carbon of the nitrile
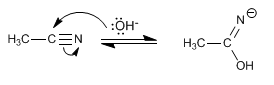
Stage 2. Protonation of nitrogen with the water in the medium.
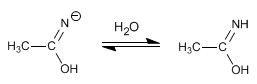
Stage 3. Tautomerism
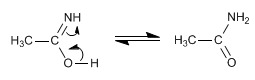
The amide formed in the last stage is hydrolyzed to sodium carboxylate and ammonia, in the reaction medium.
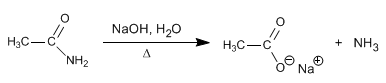
As in an acid medium, the hydrolysis reaction can be stopped in the amide, controlling the temperature and concentration of the base.