Currently, more than 30 million chemical compounds are known, of which 1 million are inorganic and the rest are organic. Chemists determine the physical and chemical properties of these substances (melting and boiling points, solubility, density...). However, the information most coveted by the chemist is the determination of the structure of the compound, what type of atoms make it up and how these atoms are bonded.
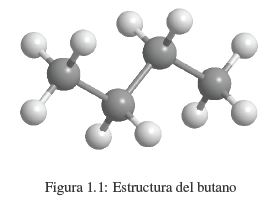
We know that a substance like butane is made up of carbon and hydrogen. Each molecule contains 4 carbon atoms and 10 hydrogen atoms, which is represented by the formula C 4 H 10 , called the molecular formula. We also know that carbon atoms are linked to form a linear chain. 3 hydrogens are attached to the first carbon, 2 hydrogens to the second carbon, 2 hydrogens to the third carbon, and the last 3 hydrogens to the fourth carbon.
How do chemists obtain this information? Unfortunately, there is no microscope capable of distinguishing atoms and seeing how they come together to form the molecule. Here are the steps to follow:
 1. Determine the molecular formula, which can be done using High Resolution Mass Spectrometry (HRMS). Although there are classical methods that allow determining the molecular formula from the percentage composition and molecular weight of the compound. At this point, we know the atoms that make up our compound and the ratio in which they participate. In the case of butane, C 4 H 10
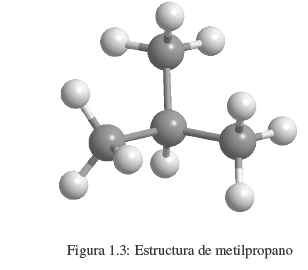
1. Determine the molecular formula, which can be done using High Resolution Mass Spectrometry (HRMS). Although there are classical methods that allow determining the molecular formula from the percentage composition and molecular weight of the compound. At this point, we know the atoms that make up our compound and the ratio in which they participate. In the case of butane, C 4 H 10 Once the molecular formula is known, we can write the possible structures of the compound. The formula C 4 H 10 is compatible with two isomers: linear butane and methylpropane.
2. Once the possible structures of our molecular formula have been considered, the analysis of the spectra will make it possible to distinguish some isomers from others to establish which of them corresponds to the problem substance.