The phenomenon by which the axial conformation of a substituted heterocycle in position 2 is stabilized is called anomeric effect. For example, 2-methoxyoxane presents a 27:73 ratio in favor of the conformation with methoxide in axial position.
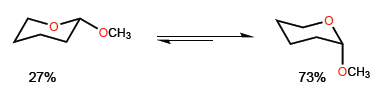
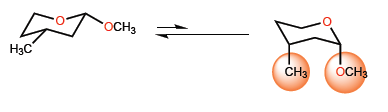
The ratio between both conformations is affected by the substituents, shifting to the right or left depending on the 1,3-diaxial interactions.
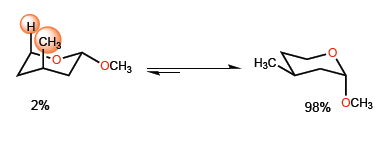
The 1,3-diaxial interaction between methyl and hydrogen shifts the equilibrium to the right.
The anomeric effect can be explained by a stabilizing interaction between the lone pair of the ring heteroatom (Y) and the C2-Z sigma bond. Such superposition implies that the orbitals involved are parallel, which happens in the conformer with Z in axial.