HETEROCYCLES - AROMATICITY
- Details
- Germán Fernández
- HETEROCYCLES - AROMATICITY
- Hits: 386
- Details
- Germán Fernández
- HETEROCYCLES - AROMATICITY
- Hits: 888
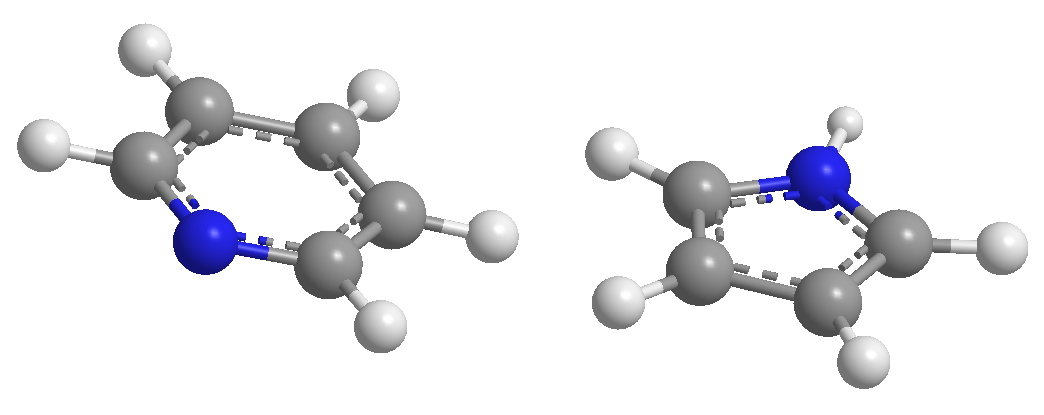
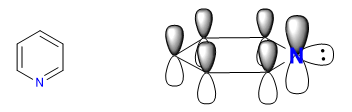
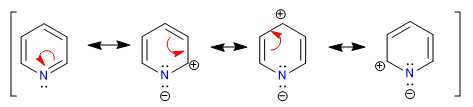
A) Heterocycles with 6 atoms and 6 p electrons.
- Details
- Germán Fernández
- HETEROCYCLES - AROMATICITY
- Hits: 458
There are several criteria to determine the degree of aromaticity of a heterocycle:
A) Link lengths.
A heterocycle is all the more aromatic the smaller the difference between the lengths of the different bonds that make up the ring. Benzene has the same length in all its carbon-carbon bonds, which makes it the most aromatic compound. The heterocycles present differences in the bond distances, being less aromatic the greater these differences are.
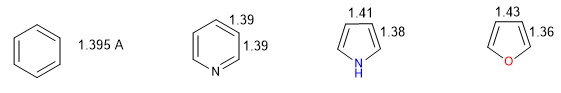
All C-C bonds in benzene measure 1.395 A, an intermediate bond distance between single (1.48 A) and double (1.34 A) bonds. In furan there is a notable difference between the length of the bonds, indicating a lower aromaticity than in pyrrole. Pyridine, on the other hand, has very similar C-C bond distances between them and similar to those of benzene, which shows greater aromaticity than pyrrole or furan.
Read more: Criteria for determining aromaticity in heterocycles
- Details
- Germán Fernández
- HETEROCYCLES - AROMATICITY
- Hits: 1352
6-Membered heteroaromatics possessing -OH or -SH groups in 2,4-positions preferentially exist in solution in the keto or thioketo forms. In solution, compounds with amino groups in 2,4-positions preferentially exist in the amino form, rather than as an imino tautomer.
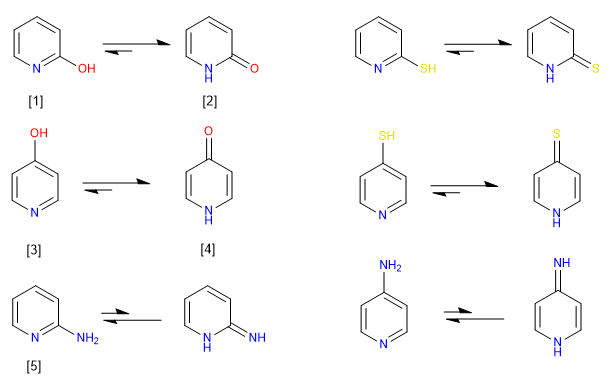
[1] 2-Hydroxypyridine
[2] 2-Pyridone
[3] 4-Hydroxypyridine
[4] 4-Pyridone
[5] 2-aminopyridine
- Details
- Germán Fernández
- HETEROCYCLES - AROMATICITY
- Hits: 782
In this section we are going to study the influence of annular stresses on the properties of heterocycles.
A) Angular stress in small heterocycles (3 and 4 members).
The natural bond angles of a sp3 carbon are 109.5º, however in three-membered cycles this angle decreases to 60o, which produces enormous tension. To alleviate this tension, the bonds between carbons are no longer straight, bending, giving rise to curved bonds called "banana bonds". The angles formed by these bonds are 104º, a more tolerable discrepancy.
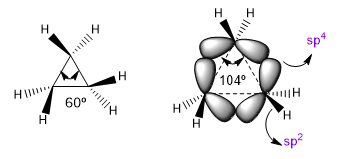
| Hybridization | Bond angle |
| sp sp2 sp3 sp4 |
180º 120º 109.5º 104º |
- Details
- Germán Fernández
- HETEROCYCLES - AROMATICITY
- Hits: 1277
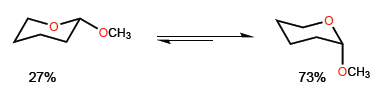
The phenomenon by which the axial conformation of a substituted heterocycle in position 2 is stabilized is called anomeric effect. For example, 2-methoxyoxane presents a 27:73 ratio in favor of the conformation with methoxide in axial position.