HETEROCYCLE NOMENCLATURE
- Details
- Germán Fernández
- HETEROCYCLE NOMENCLATURE
- Hits: 22919
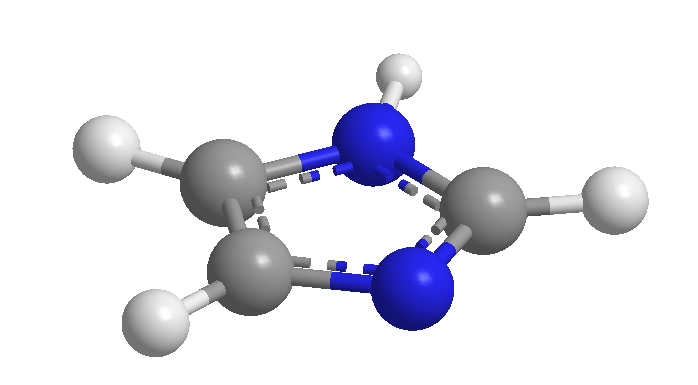
We must take into account that many heteromonocycle compounds have been known for more than 100 years, and that they were isolated from natural products.  In the absence of a systematic nomenclature, they were originally given common names related to their origin, with some property, ect. These names are so ingrained that, even if there are systematic alternatives, the IUPAC does not aspire to their use, but rather recommends the former.
In the absence of a systematic nomenclature, they were originally given common names related to their origin, with some property, ect. These names are so ingrained that, even if there are systematic alternatives, the IUPAC does not aspire to their use, but rather recommends the former.
- Details
- Germán Fernández
- HETEROCYCLE NOMENCLATURE
- Hits: 30247
The systematic name of the heterocycles is built from a prefix that indicates the type of heteroatom it contains, and a suffix that depends on the size of the cycle. 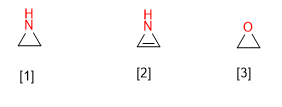
[1] Aziridine
[2] Azirine
[3] Oxirane
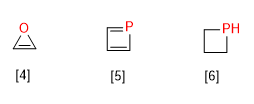
[4] Oxirene
[5] Phosphete
[6] Phosphethane
- Details
- Germán Fernández
- HETEROCYCLE NOMENCLATURE
- Hits: 9168
Let us now consider the cases of unsaturated heterocycles (with at least one double bond), but which do not have the maximum number of double bonds. They are named as if they were completely unsaturated, putting the dihydro, tetrahydro, hexahydro, etc. particles before the name. (always indicative of an even number of hydrogens, as corresponds to formal hydrogenations of one, two, three, etc. double bonds). Keep in mind that formal hydrogenations may have occurred on neighboring or remote carbon atoms in the heterocycle.
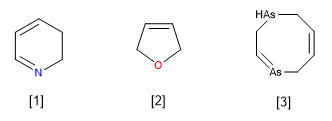
A pyridine heterocycle missing a double bond at the 2,3-position. (2,3-Dihydropyridine)
Furan heterocycle missing a double bond. (2,5-dihydrofuran).
![]() Note that dihydros can be used with non-adjacent carbons.
Note that dihydros can be used with non-adjacent carbons.
Diarsocine heterocycle missing two double bonds. (1,2,5,8-Tetrahydro-1,4-diarsocine)
- Details
- Germán Fernández
- HETEROCYCLE NOMENCLATURE
- Hits: 9508
Observe the following examples. In all of them there is the maximum number of non-cumulative double bonds, and there is also a ring atom linked to neighboring atoms only by single bonds. If that atom also has one (or two) hydrogen atoms, that hydrogen (or one of the two) receives the name of indicated hydrogen, and must be stated in the name.
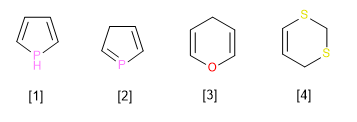
[1] A pyrrole heterocycle that has the largest possible double bonds, but lacks a double bond on the phosphorous atom. This fact must be indicated in the name through the hydrogen indicated in position 1. (1H-Pyrrole)
[2] Pyrrole heterocycle with the largest double bonds. The lack of double bond in position three should be reflected in the name (3H-Pyrrole)
[3] Pyrane heterocycle with hydrogen indicated in position 4. (4H-Pyrane)
[4] Dithiine heterocycle, with maximum double bonds, but with two saturated carbons that should be reflected in the name (2H,4H-1,3-dithiine)
- Details
- Germán Fernández
- HETEROCYCLE NOMENCLATURE
- Hits: 7453
- Details
- Germán Fernández
- HETEROCYCLE NOMENCLATURE
- Hits: 8634
When a heterocycle acts as a substituent, it is named following the rules that we already know, and then the final vowel of the name is deleted, and it is replaced by “n-il”, where n is the locator of the atom of the heterocycle that is attached to the rest of the molecule. If there is a choice, the lowest possible value is given.
- Details
- Germán Fernández
- HETEROCYCLE NOMENCLATURE
- Hits: 8754









