REACTION MECHANISM
Radical halogenations take place in three stages called: initiation, propagation and termination:
Initiation stage
In the first step of the reaction, the homolytic cleavage of the Cl-Cl bond occurs. This is achieved with heat or by absorbing light.
![]()
First stage of propagation
It is a slightly endothermic stage that consists of the subtraction of a hydrogen from methane by the chlorine radical formed in the previous stage, generating the methyl radical.
![]()
Second stage of propagation
During it, the methyl radical abstracts a chlorine atom from one of the initial molecules, giving chloromethane and a new chlorine atom. Said atom returns to the first stage of propagation and the whole process is repeated.
![]()
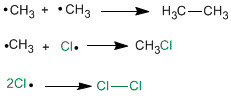
Termination stage
It takes place when the reagents are exhausted, then the radicals that are in the middle unite with each other.