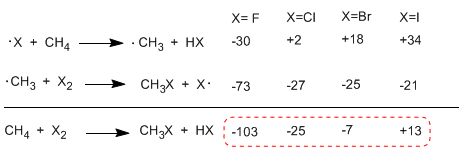THEORY HALOGENATION REACTIONS
- Details
- Germán Fernández
- THEORY HALOGENATION REACTIONS
- Hits: 902
 Alkanes react with halogens through radical mechanisms. Said reaction supposes the substitution of one or several hydrogens of the alkane by halogens.
Alkanes react with halogens through radical mechanisms. Said reaction supposes the substitution of one or several hydrogens of the alkane by halogens.
Mechanism of radical halogenation
The radical halogenation mechanism consists of three stages: initiation, propagation and termination. In initiation, the halogen molecule breaks homolytically, generating radicals. In the propagation stage, the substitution of hydrogens of the alkane by halogens occurs. When the reactants are exhausted, the radicals in the middle unite with each other, producing the termination stage.
Halogen reactivity
The first stage of propagation determines the rate of the reaction. For fluorine, this stage has a low activation energy, which makes fluorine the most reactive halogen. In the case of iodine, the activation energy is very high and the reaction does not take place. Order of reactivity in radical reactions: $F_2>Cl_2>Br_2>I_2$. In short, iodine is unreactive in radical halogenation and fluorine reacts violently.
Polyhalogenations
The halogenation reaction is difficult to stop, since the halogenated product is more reactive than the starting alkane. To avoid this problem, called polyhalogenations, excess alkane is used.
Radical stability
The mechanism of these reactions occurs with the formation of an intermediate called a radical whose stability depends on the number of substituents that surround the carbon that contains the lone electron. The radicals formed in the propagation step are stabilized by hyperconjugation. The order of stability of the radicals is given by: tertiary> secondary> primary.
- Details
- Germán Fernández
- THEORY HALOGENATION REACTIONS
- Hits: 48326
REACTION MECHANISM
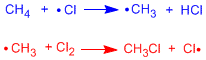
Radical halogenations take place in three stages called: initiation, propagation and termination:
Initiation stage
In the first step of the reaction, the homolytic cleavage of the Cl-Cl bond occurs. This is achieved with heat or by absorbing light.
![]()
- Details
- Germán Fernández
- THEORY HALOGENATION REACTIONS
- Hits: 24063
We are going to represent in an energy diagram the two stages of propagation of the halogenation of methane.
The first stage of propagation is the one that limits the speed of the process, it has the highest activation energy. The diagram represents reactants, products, intermediates, and transition states for the radical halogenation of methane.
- Details
- Germán Fernández
- THEORY HALOGENATION REACTIONS
- Hits: 19777
When comparing the enthalpies of the propagation stages of the different halogens, important differences in the exchanged heats are observed. In the case of fluorine, both steps are exothermic (even the abstraction of hydrogen) with a global energy balance of -103 Kcal/mol. The activation energies of the transition states in this reaction are very low, making it the most reactive halogen.
At the other extreme of reactivity is iodine, whose reaction is endothermic and does not take place.
Order of reactivity F2>Cl2> Br2> I2
The following table shows the heat exchanged in the propagation stages for the halogenation of methane.
The reaction is strongly exothermic in the case of fluorine (halogen more reactive) while in the case of iodine it is endothermic at 13 Kcal/mol.
- Details
- Germán Fernández
- THEORY HALOGENATION REACTIONS
- Hits: 19711
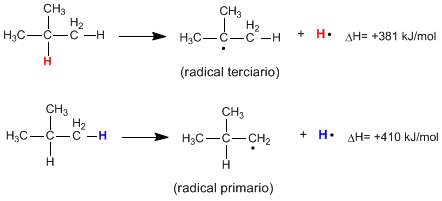
Homolytic breaking of the CH bond of an alkane produces alkyl radicals and free hydrogen atoms. The energy necessary for this breakage to occur is called dissociation energy and it is lower the more stable the radical formed is.
As can be seen from the following reactions, the energy required to break a primary CH bond is much higher than that required to break a tertiary CH bond.
- Details
- Germán Fernández
- THEORY HALOGENATION REACTIONS
- Hits: 24502
In propane there are two types of non-equivalent hydrogens that can be replaced by halogen, obtaining 1-chloropropane and 2-chloropropane.
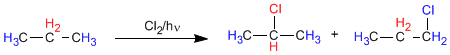
The proportion in which these products are obtained depends on two factors: the number of hydrogens to be replaced by halogen and the stability of the radical formed.