Unimolecular deletions (E1) compete with unimolecular nucleophilic substitution (SN1), forming alkenes.
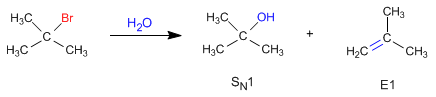
The nucleophilic behavior of water generates the substitution product (S N 1), while the basic character gives rise to the elimination product (E1).
The mechanism of the E1 reaction occurs with the following steps:
Stage 1. Ionization of the substrate
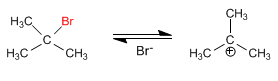
Stage 2. The water acts as a base, removing the proton from the carbon next to the positive one (beta carbon), to form the alkene.
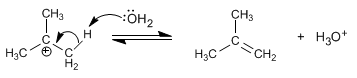
If water acts as a nucleophile, it gives rise to products of the SN1 type. The low basicity of water means that the substitution product is in the majority.









