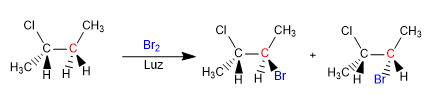
We are going to see how chemical reactions can introduce chirality in molecules, obtaining products in the form of racemic mixtures or mixtures of diastereoisomers.
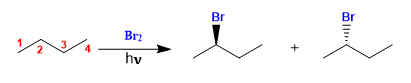
Butane halogenates in the presence of bromine and light, at carbon 2, to form a mixture of enantiomers. The radical formed presents enantiotopic faces, which are halogenated with equal probability, giving rise to a racemic mixture (enantiomers in equal proportion).
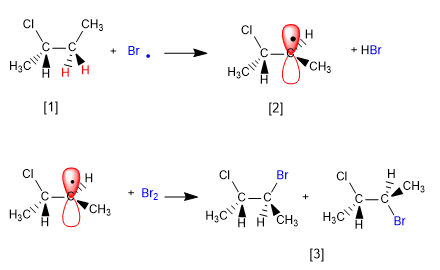
The mechanism of this reaction consists of three stages: initiation, propagation and termination. Propagation is the step that determines the stereochemistry of the final product.
Butane halogenation
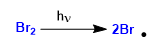
Stage 1. Initiation
Stage 2. Propagation
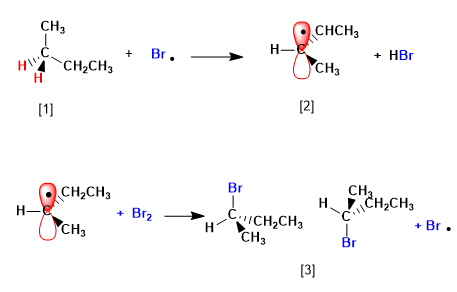
[1] H (Enantiotopic Hydrogens)
[2] Radical with enantiotopic faces
[3] Pair of enantiomers
The product is obtained as a racemic mixture, due to the formation of a planar radical that is halogenated on both faces. The enantiotopic hydrogens are chemically equivalent and are subtracted by bromine at the same rate.