Synthesis of local anesthetics derived from aminobenzoic acid
Local anesthetics derived from m-aminobenzoic acid
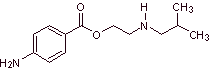
The most representative drugs of this group are
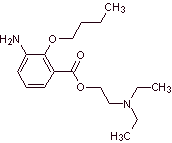
MOb 07: Metabutoxycain , marketed under the name primacaine , is another local anesthetic used in dentistry. Propose a synthesis design for this drug, starting from simple and affordable materials. |
|
Retrosynthetic analysis : Acyl-oxygen cleavage generates an amino alcohol cleavable to secondary amine and epoxide; the other precursor invites to prepare its carboxyl group by hydrolysis of a nitrile group, which will be placed on the benzene ring by the Sandmeyer reaction. The selective reduction of only one of the nitro groups is carried out with ammonium polysulfide or also with Na 2 S.
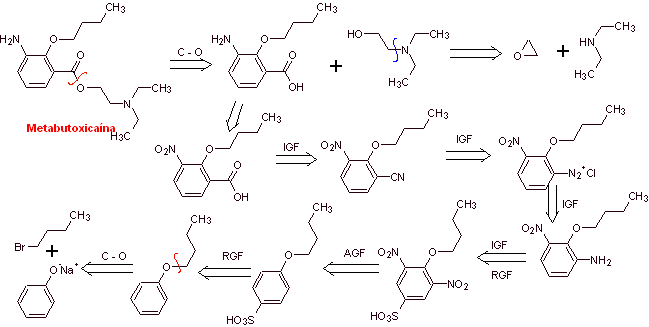
Synthesis of
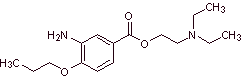
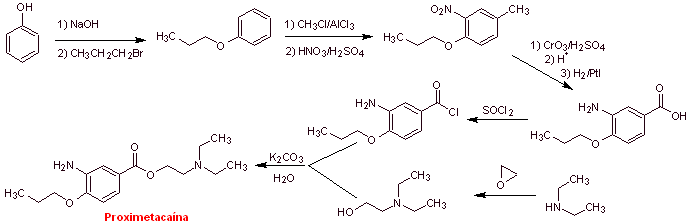
MOb 08: Proximethacaine (INN) or proparacaine , known by the trade names Alcaine, Ak-Taine, and others, is a topical anesthetic drug from the amino ester group. It is indicated for use as an ophthalmic anesthetic to reduce pain and discomfort in the eye. Propose a synthesis design for this anesthetic, starting from simple and affordable materials. |
|
Retrosynthetic analysis : The initial acyl-oxygen disconnection of proxymethacaine, again leads to an m-aminobenzoic acid, with an alkoxide substituent in the para position and an aminoalcohol that is formed from the corresponding amine and epoxide.
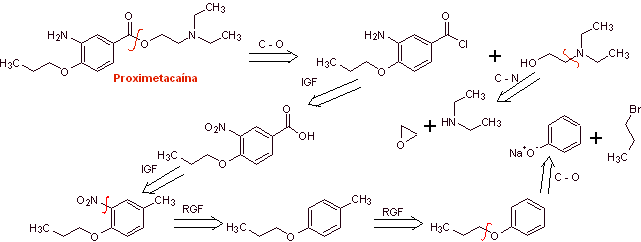
Synthesis of proximethacaine :
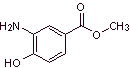
MOb 09: Orthocaine is another local anesthetic, propose a synthesis plan for this molecule, from simple and affordable materials. |
|
Retrosynthetic analysis : It begins with the disconnection of the acyl-oxygen bond of the ester function, to form the carboxyl group From the oxidation of the methylene group, the amino group must be deactivated, its precursor nitro group and OH must be protected as a benzyl ether. It is impossible to think of oxidizing the methyl group of benzene while the amino and hydroxyl groups are present, which are much more reactive and would form quinones.
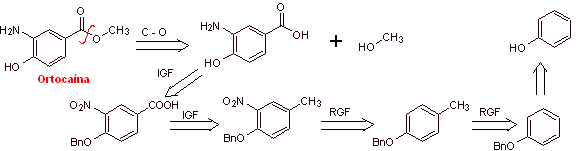
Orthocaine synthesis :
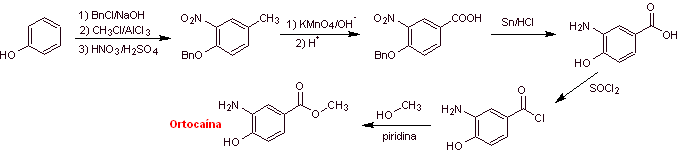
Local anesthetics derived from p-aminobenzoic acid
The most abundant local anesthetics belong to this type of compound, of which the following can be mentioned:
Benzocaine, procaine (novocaine), butetamine, propazine, chloroprocaine, risocaine, propoxycaine, dimethocaine (larocaine), butamben (butyl aminobezoate), butacaine, isobutamben, oxybuprocaine (benoxynate), tetracaine (amethocaine), tricaine, isobucaine, meprilcaine, metabulethamine , naepain, procainamide.
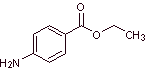
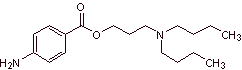
MOb 10 : Benzocaine is a local anesthetic for external use (topical anesthesia). It is the active ingredient in many anesthetic ointments. Propose a synthesis plan from simple materials for this drug. |
|
Retrosynthetic analysis : An alternative synthesis for benzocaine that avoids the nitration of toluene, due to the low yield that it causes in the synthesis, due to the fact that the reaction yields only 38% of the nitrated isomer in the para position, may be the following:
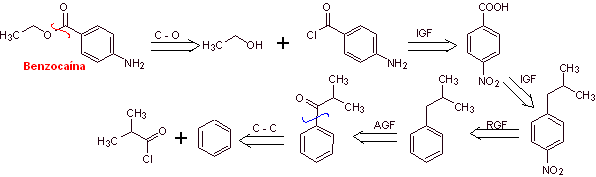
Synthesis of benzocaine : The starting materials are acetylene and benzene.
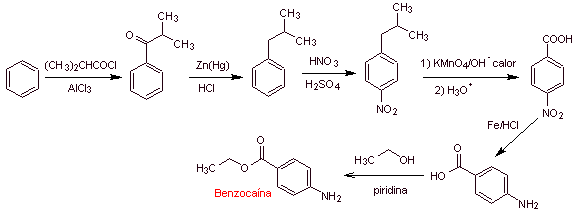
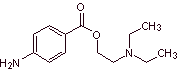
MOb 11: Procaine (novocaine) is a local anesthetic, mainly used in intramuscular injections and is also used in dentistry. It was synthesized in 1905 by Alfred Einhorn (1856-1917). Propose a synthesis design, for this drug, from simple and affordable materials. |
|
Retrosynthetic analysis : The most adequate initial disconnection is that of acyl-oxygen. The required amino alcohol is formed by opening an epoxide with the secondary amine. Para-aminobenzoic acid requires the alkylation of benzene, through a previous acylation, reduction of the carbonyl group, to guarantee the isomer for its nitration. There are some reports of a direct lacylation with propenyl. however, the cumene, which is the product formed, does not produce the carboxylic group by oxidation, but a phenol is obtained.
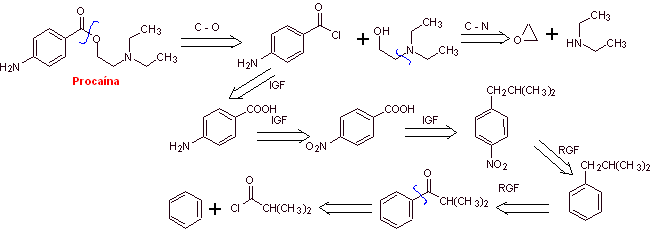
Procaine synthesis :
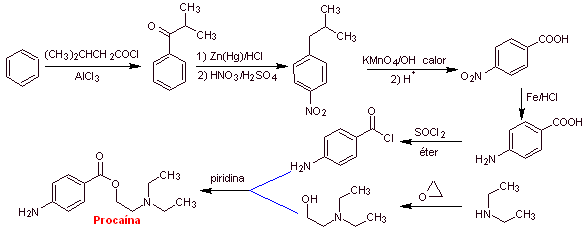
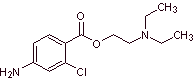
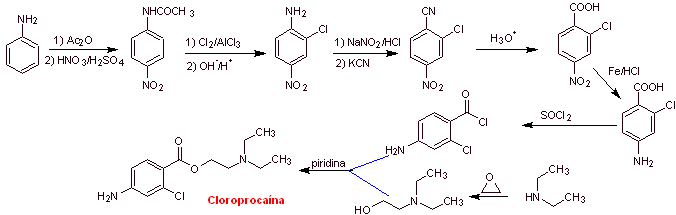
MOb 12 : Chloroprocaine (trade name Nesacaine, Nesacaine-MPF), in the hydrochloride salt form of the brand names mentioned above, is a local anesthetic. Propose a synthesis design from simple and affordable materials |
|
Retrosynthetic analysis : The disconnections used in the previous syntheses guide the present one. However, it is necessary to highlight that para-aminobenzoic, chlorinated in its ortho position, requires a strategy that guarantees the formation of this isomer.
Here, a second alternative is presented, for the formation of para-aminobenzoic acid that does not use the isopropyl group,
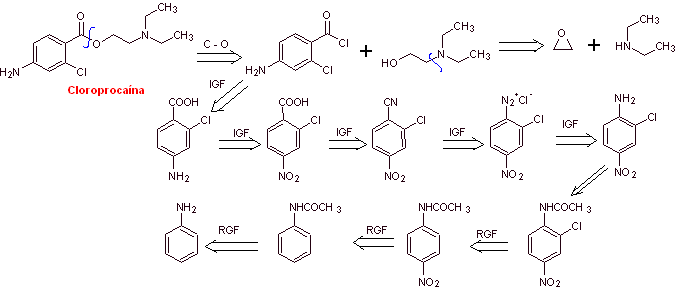
chloroprocaine synthesis
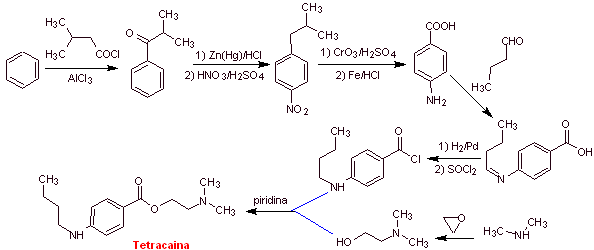
MOb 13. Tetracaine (also known as amethocaine, pontocaine, and dicaine ) is a potent local anesthetic. It is mostly used topically in ophthalmology. Propose a plan for its synthesis, from simple and affordable materials |
|
Retrosynthetic analysis : Tetracaine is an amino ester, derived from p-aminobenzoic acid
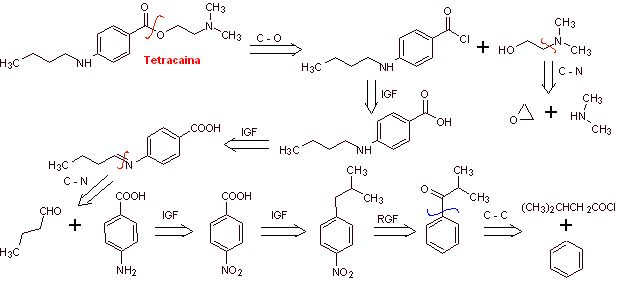
Tetracaine synthesis: Toluene, diethyl malonate and ethyl alcohol can be used as simple starting materials.
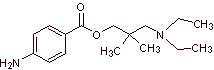
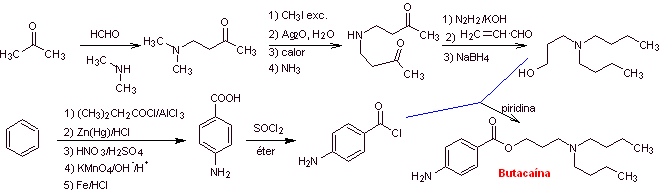
MOb. 14 . Butacaine is another local anesthetic, to propose a synthesis design for it, from simple materials. |
|
Retrosynthetic analysis : The initial disconnection of the acyl-oxygen bond from the ester group promotes the presence of p-aminobenzoic acid and an aminoalcohol as precursor molecules. p-Aminobenzoic acid is prepared from benzene via isopropylbenzene for the reasons explained abundantly up to now.
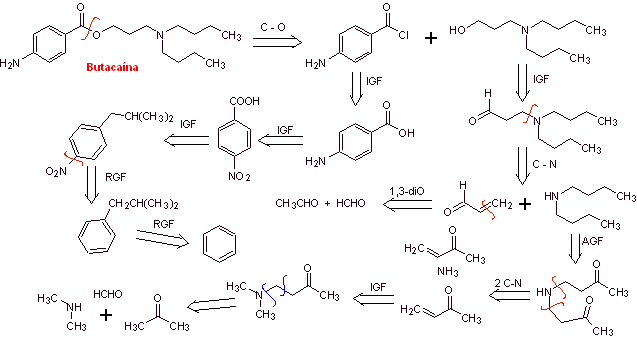
Butacaine synthesis : Acetone and benzene are the simple and affordable starting materials
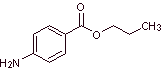
MOb 15: Risocaine ( propyl 4-aminobenzoate ) is a local anesthetic. Propose a synthesis route, for this drug, from simple and affordable materials |
|
Retrosynthetic analysis : The acyl-oxygen disconnection generates aminobenzoic acid and propanol as precursor molecules, which are prepared by one of the paths previously explained and discussed for ac. p-aminobenzoic. The alcohol is prepared with the Grignard reagent.
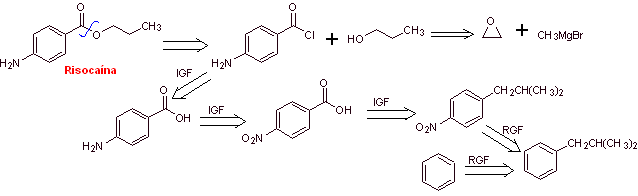
Synthesis of
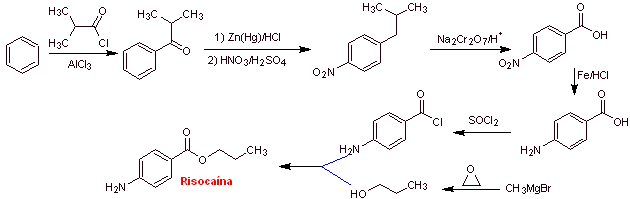
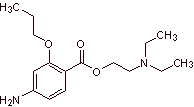
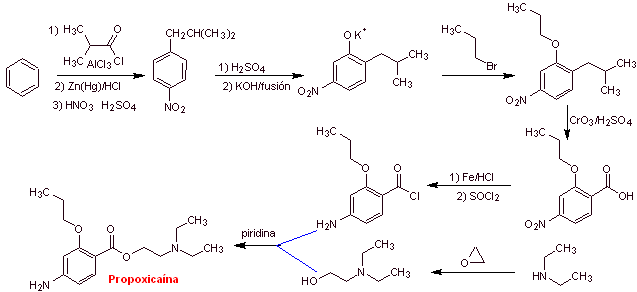
MOb 16 : Propoxycaine , is a local anesthetic. Propose a synthesis design, for this molecule, from simple and affordable materials. |
|
Retrosynthetic analysis : The acyl-oxygen disconnection is the most indicated, to start the analysis process
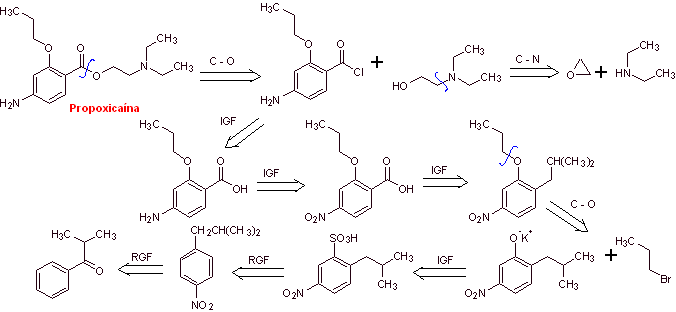
Propoxycaine synthesis :
MOb 17: Dimethocaine , also known as larocaine , is a local anesthetic with stimulant properties that some studies show to be almost as potent as cocaine. Propose a synthesis plan for this molecule. |
|
Retrosynthetic analysis : The acyl-oxygen disconnection generates two precursor molecules. p-Aminobenzoic acid will be prepared from isopropylbenzene. The aminoalcohol, due to the number of methyl groups, can be related to the multialkylation of diethyl acetoacetate, which by decarboxylation and reduction will generate the necessary aminoalcohol.
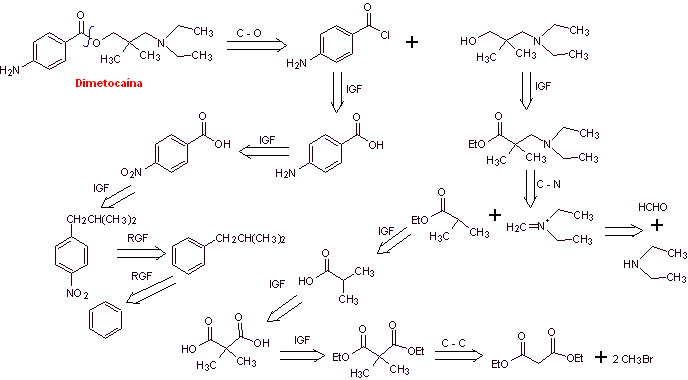
Dimethocaine synthesis :
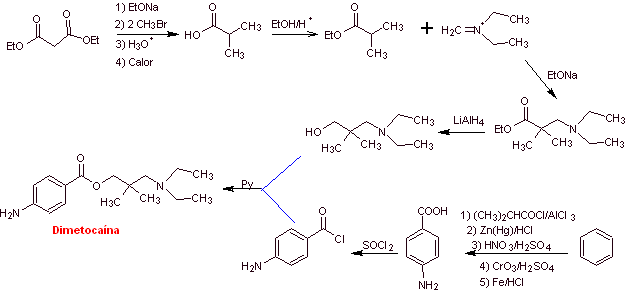
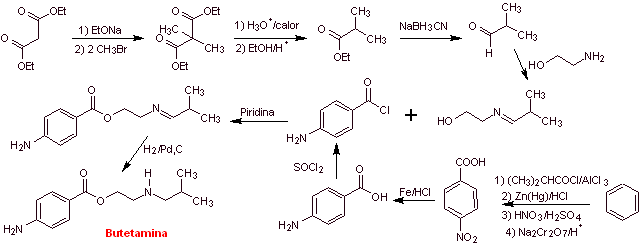
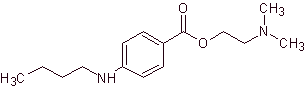
MOb 18: Butetamine is a local anesthetic. What is a possible synthesis route for it? The synthesis must start from simple and affordable materials. |
|
Retrosynthetic analysis: The secondary amine in the butetamine structure prevents the first disconnection from being carried out by the acyl-oxygen bond of the ester. A synthesis can be postulated according to the following disconnection scheme:
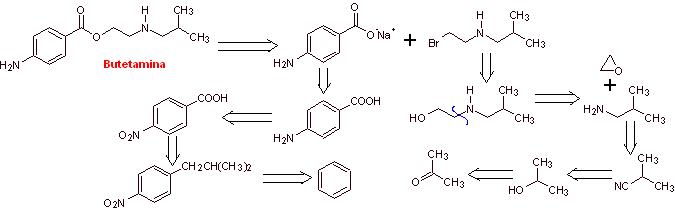
The reactions have already been discussed in the synthesis of the previous anesthetics, for which another strategy will be used, whose benefits must be duly evaluated in light of the generally accepted reaction mechanisms.
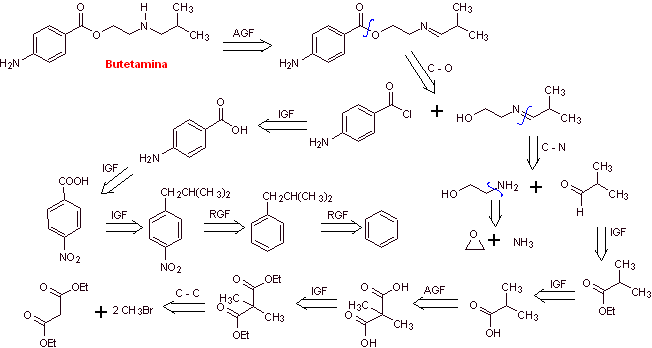
Butetamine synthesis :