Synthesis of BENZODIAZINES
(By the method of disconnections)
The structures of benzodiazines are found in many alkaloids, mainly as a quinazolone ring system. The other derivatives of benzodiazine, such as cinnolines, quinoxalines and phthalizines, are also an important part of many drugs with a spectrum of significant use, which makes them, in general, very important in organic synthesis and particularly in pharmacochemistry. Thus, they can be found as anti-inflammatory, antihypertensive, antibacterial, analgesic, antibiotic, etc.
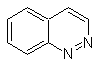
cinnoline |
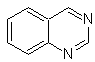
quinazoline | |
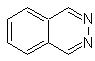
phthalizine |
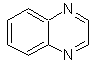
Quinoxaline |
Synthesis of the Cinnolines
According to the structure that cinnoline presents, there are the following options for its synthesis:
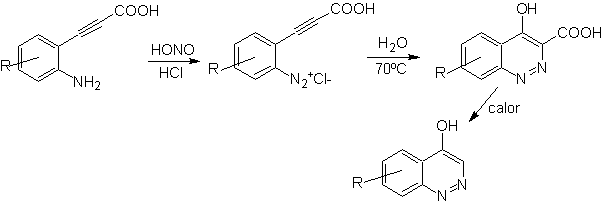
![]() von Richter synthesis:
von Richter synthesis:
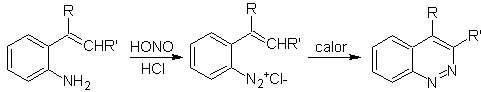
![]() Widman–Stoermer synthesis:
Widman–Stoermer synthesis:
Propose a synthesis plan for the following molecule : | MOb 114
|
MOb 114 . Retrosynthetic analysis.
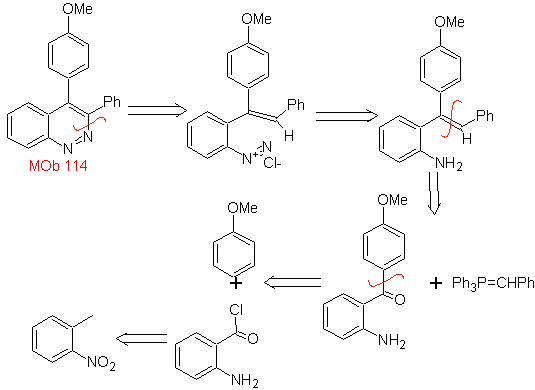
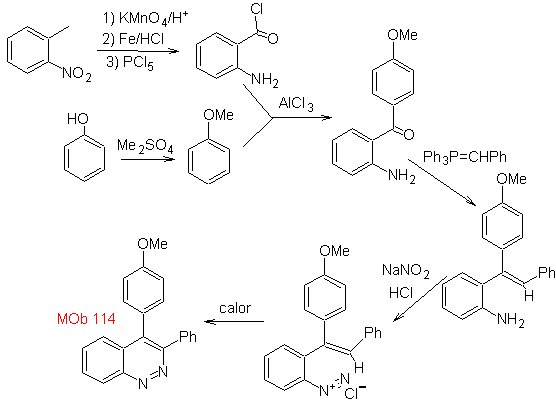
Synthesis The formation of
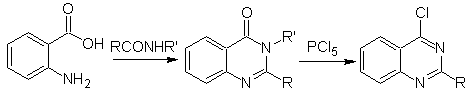
Synthesis of quinazolines.
Inside Of the classic syntheses for quinazolines, the following can be mentioned:
Yo.
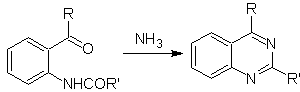
Niementowski synthesis:
ii.
Other variants:
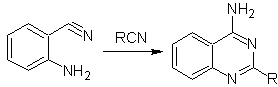
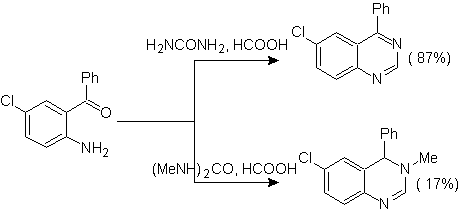
iii.
More reactions that form good quinazoline precursors:
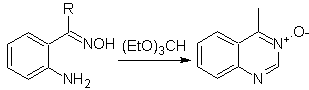
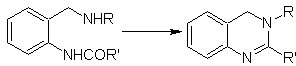
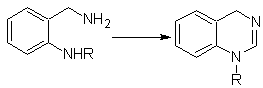
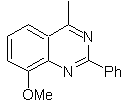
Propose a synthesis design for the following quinazoline: | MOb 115
|
MOb 115. Retrosynthetic analysis. The disconnection starts
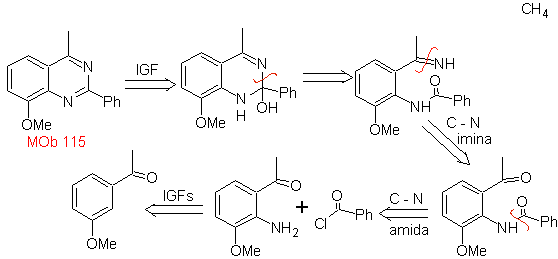
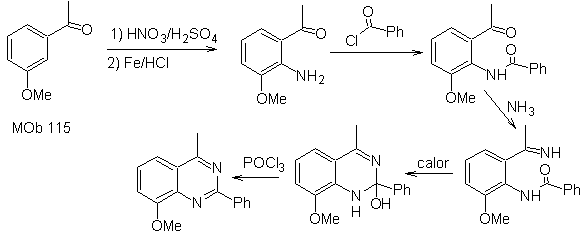
Synthesis It can be started from benzene or 3-methoxyacetophenone, easily obtainable from benzene and continue with its nitration, to introduce the amino group, which, transformed into amide and ammonia, allows us to reach
Synthesis of phthalizines.
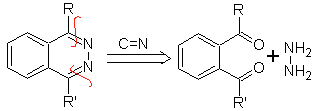
The limitations of this synthesis are related to those that occur in the preparation of the aromatic 1,2-dicarbonyl compound.
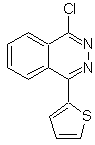
Propose a synthesis design for the following molecule: | MOb 116
|
MOb 116. Retrosynthetic analysis. With the necessary IGFs on
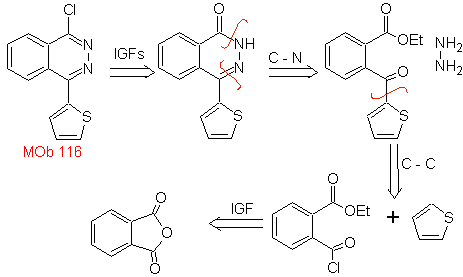
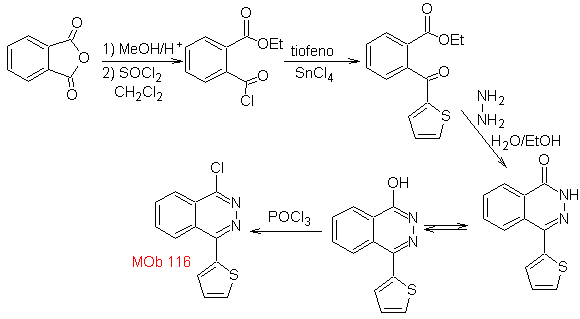
Synthesis. Phthalic anhydride is a good starting material because of its low cost and easy preparation. The rest of the reactions allow us to show the use of hydrazine. POCl 3 is used as a direct agent to displace OH and introduce Cl, for the synthesis of
Synthesis of Quinoxalines
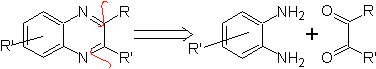
Quinaxolines are possibly the easiest benzodiazine isomers to prepare. Thus, the synthesis of Quinaxolines could be faced according to the criteria followed in the initial disconnection of the molecule to be synthesized and the presence of substituents in both rings.
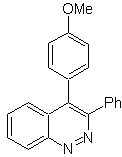
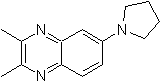
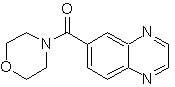
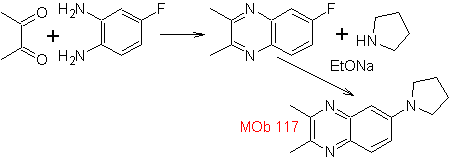
Synthesize the following molecules: | MOb 117
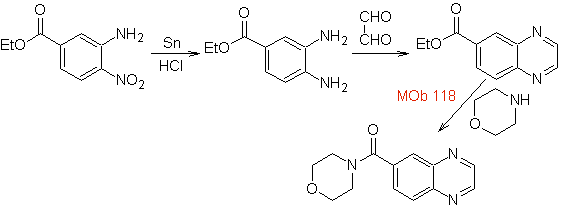
| MOb 118
|
MOb 117. Retrosynthetic analysis . The fluorine, of
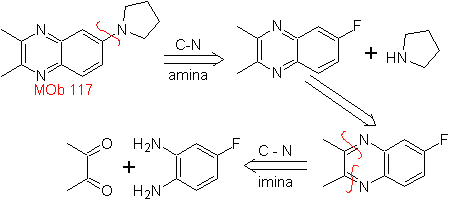
Synthesis. The diketonic compound and the aromatic diamino present no difficulty in their preparation, as starting materials for
Ethyl 3-amino-4-nitrobenzoate can still be cut off to aniline as starting material.
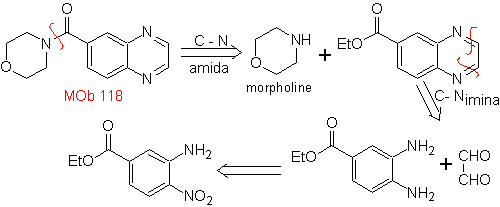
synthesis . From aniline, the intermediate ethyl 3-amino-nitrobenzoate is formed, which combines with oxaldehyde and later with morpholine to form