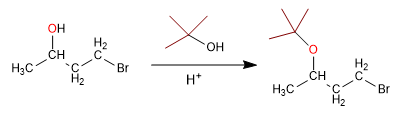
Alcohols can be protected by transforming them into ethers. This process is carried out by reacting the alcohol to be protected with tert-butanol in a sulfuric acid medium. The deprotection takes place in an aqueous acid medium. Let's see an example:
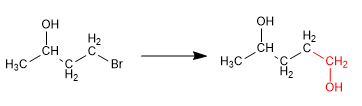
In this synthesis it is necessary to form a carbon-carbon bond using organometallic reagents, incompatible with alcohol. Thus, the alcohol must be previously protected to avoid the decomposition of the organometallic.
Stage 1. Protection from alcohol
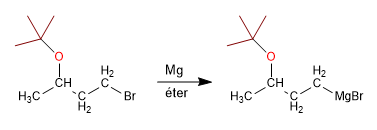
Stage 2 . Organometallic formation
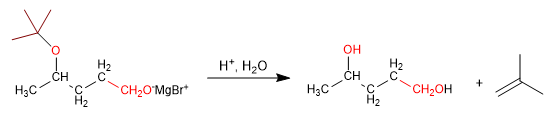
Stage 3. Reaction of magnesium with methanal
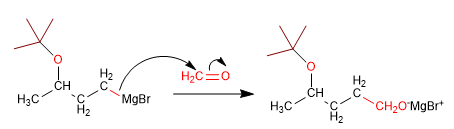
Stage 4. Deprotection and protonation of the alkoxide.