Cycloalkanes nomenclature 
Cycloalkanes are named with the prefix cyclo- followed by the name of the alkane with the same number of carbons. Cycloalkanes exhibit cis/trans isomerism. When the substituents are on the same face of the molecule, they are said to be cis; when they meet on opposite sides, they are said to be trans.
Physical properties
They have higher melting and boiling points than the corresponding alkanes of the same number of carbons. The rigidity of the ring allows a greater number of intermolecular interactions, which it is necessary to break through the input of energy, to pass the molecules to the gas phase.
Annular tension
Small size cycloalkanes (cyclopropane, cyclobutane) present significant stress due to bond angles and eclipsing. Larger cycloalkanes such as cyclopentane and cyclohexane are nearly stress-free.
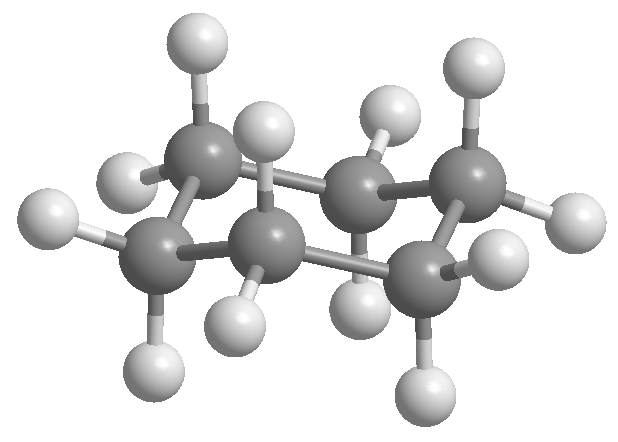
Conformational isomers in cyclohexane
Cyclohexane is arranged in the form of a chair to avoid eclipsing between hydrogens. The chair form of cyclohexane contains two types of hydrogens; the axial ones that are located perpendicular to the plane of the molecule and the equatorial ones placed in the same plane.
Equatorial-axial equilibrium in substituted cyclohexanes
Cyclohexane presents a conformational equilibrium that interconverts equatorial hydrogens to axial ones and vice versa. When a cyclohexane is substituted, the conformation that places the most groups in the equatorial position is the most stable, finding the conformational equilibrium shifted towards said conformation.