Cycloalkanes are alkanes that have the chain ends joined together, forming a cycle. They have two fewer hydrogens than the alkane from which they are derived, which is why their molecular formula is CnH2n . They are named using the prefix cyclo followed by the name of the alkane.
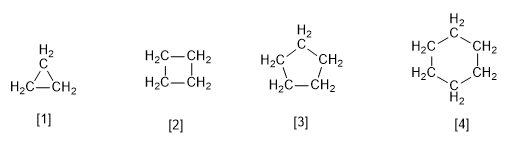
[1] Cyclopropane
[2] Cyclobutane
[3] Cyclopentane
[4] Cyclohexane
It is common to represent molecules indicating only their skeleton. Each vertex represents a carbon bonded to two hydrogens. 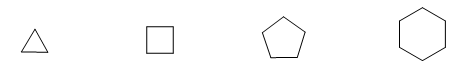
The IUPAC rules for naming cycloalkanes are very similar to those studied for alkanes.
Rule 1.- In cycloalkanes with a single substituent, the cycle is taken as the main chain of the molecule. Cycle numbering is unnecessary.
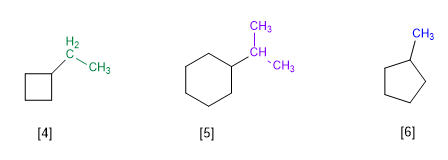
[4] Ethylcyclobutane
[5] Isopropylcyclohexane
[5] Methylcyclopentane
If the side chain is complex, it can be taken as the main chain of the molecule and the cycle as a substituent. Cycloalkanes as substituents are named by changing the ending –ane to –yl.
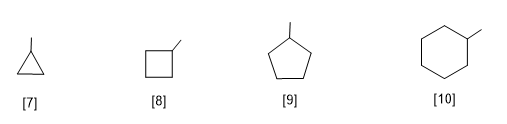
[7] Cyclopropyl
[8] Cyclobutyl
[9] Cyclopentyl
[10]Cyclohexyl
Rule 2.- If the cycloalkane has two substituents, they are named in alphabetical order. The cycle is numbered starting with the substituent that comes before it in the name.
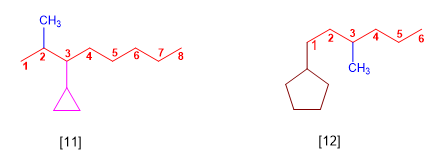
[11] 3-Cyclopropyl-2-methyloctane
[12] 1-Cyclopentyl-3-methylhexane
Rule 3.- If the ring has three or more substituents, they are named in alphabetical order. The numbering of the cycle is done in such a way that the lowest locants are given to the substituents.

[14] 1-Bromo-2-ethyl-4-methylcyclopentane
[15] 2-Bromo-1-chloro-3-methylcyclopentane
In case of obtaining the same locants when numbering starting with different positions, the alphabetical order is taken into account.
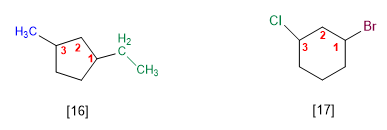
[16] 1-Ethyl-3-methylcyclopentane
[17] 1-Bromo-3-chlorocyclohexane









