NOMENCLATURE OF CONDENSED HETEROCYCLES
- Details
- Germán Fernández
- NOMENCLATURE OF CONDENSED HETEROCYCLES
- Hits: 548
Fused heterocycles are formed by two cycles that share a bond, the condensation bond. One of the heterocytes is considered principal, while the other is called annexed. The name is built starting with that of the attached heterocycle, followed by a bracket where the bond by which both cycles condense is specified and ending with the name of the base heterocycle.
Read more: General concepts of condensed heterocycle nomenclature
- Details
- Germán Fernández
- NOMENCLATURE OF CONDENSED HETEROCYCLES
- Hits: 707
The following criteria apply until one unequivocally decides:
1. A compound containing nitrogen.
2. The component containing the highest priority heteroatom (without nitrogen).
3. The component that contains the largest of the rings.
4. The component containing the largest number of heteroatoms of any type.
5. The component containing the greatest variety of heteroatoms.
6. The component containing the largest number of most preferred heteroatoms.
7. The component that, before condensation, has minor locants for its heteroatoms.
8. Rule 7, considering the heteroatoms in order of priority.
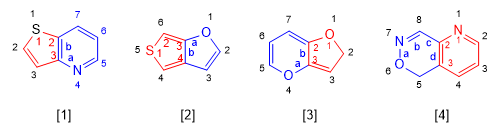
In the following fused heterocycles the base component is represented in blue.
- Details
- Germán Fernández
- NOMENCLATURE OF CONDENSED HETEROCYCLES
- Hits: 603
Assign locant 1 to an atom adjacent to a bridgehead, and move further away from it so that lower locants are given to:
1. The heteroatoms together.
2. The heteroatoms according to priority.
3. The carbon atoms that are in the fusion position.
4. The indicated hydrogens. In peripheral numbering, the bridgehead carbons are not numbered, although they are when heteroatoms coincide in that position.
- Details
- Germán Fernández
- NOMENCLATURE OF CONDENSED HETEROCYCLES
- Hits: 440
Their objective is to determine the positions by which both cycles condense. The base heterocycle is literate, each bond is assigned a letter, starting from the bond that joins atoms 1,2, to which the letter a is associated, until reaching the condensation bond, whose letter appears inside the bracket in the heterocycle name. The attached heterocycle is numbered from atom 1 to the condensation carbons, by the shortest path. The numbers of these condensing carbons are entered in the square bracket next to the literal letter. [1,2-b] indicates that the fusion bond contains the 1,2 carbons of the appended heterocycle and the b bond of the base.
Let's see an example.
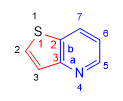
Base: Pyridine (Rule 1. component with nitrogen)
Annex: Thiophene (thiene)
Annexed numbering: starts at the sulfur and goes towards the C. bridge.
Base literation: it begins in the nitrogen and goes towards the C. bridge.
Peripheral numbering: Start at sulfur to give heteroatoms the lowest locants. (rule 1)
Name: Thieno[3,2-b]pyridine
Read more: Literation of the base heterocycle and numbering of the annex
- Details
- Germán Fernández
- NOMENCLATURE OF CONDENSED HETEROCYCLES
- Hits: 950
Molecule 1.
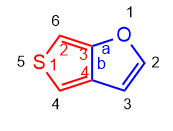
Base: Furan (Rule 2. component with higher priority heteroatom)
Annex: Thiophene (thiene)
Annexed numbering: starts at the sulfur and goes towards the C. bridge.
Literature base: it begins in the oxygen and goes towards the C. bridge.
Peripheral numbering: start at oxygen to give the lowest locants to the heteroatoms. (rule 1)
Name: Thieno [3,4-b] furan









