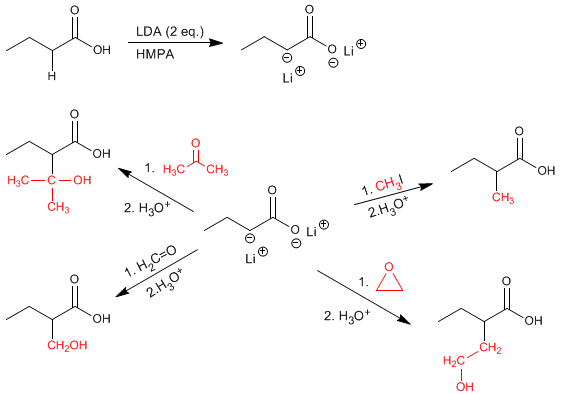
The a -hydrogens of carboxylic acids are acidic and can be removed using strong bases such as LDA.
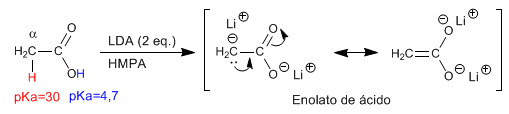
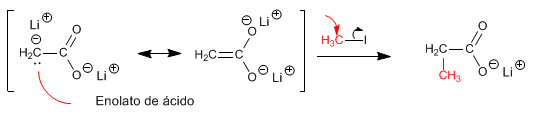
The first equivalent of LDA strips the hydrogen from the hydroxyl group (pKa = 4.7), forming the carboxylate. The second equivalent of LDA deprotonates the a- carbon, forming the acid enolate.
A highly polar solvent (HMPA) is used to stabilize the enolate.
Acid enolates are good nucleophiles and attack through carbon an important variety of electrophiles (primary haloalkanes, epoxides, aldehydes, ketones...)
Other examples on the reactivity of acid enolates