CARBOHYDRATES THEORY
- Details
- Germán Fernández
- CARBOHYDRATES THEORY
- Hits: 2060
The simplest carbohydrates are sugars or saccharides, they are polyhydroxylated aldehydes or ketones. They are classified as aldoses, if they have the aldehyde function, and ketoses, if they have the ketone function.
The aldoses are classified depending on the number of carbons in: aldotrioses, aldotetroses, aldopentoses and aldohexoses.
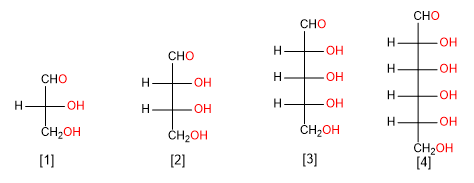
Aldose of three carbon atoms (aldotriose)
Aldose of four carbon atoms (aldotetrose)
Aldose of five carbon atoms (aldopentose)
Aldose of six carbon atoms (aldohexose)
- Details
- Germán Fernández
- CARBOHYDRATES THEORY
- Hits: 34254
The following table compiles the natural monosaccharides that contain an aldehyde group, called aldoses. The table is built starting with the smallest aldose, glyceraldehyde (aldotriose). Erythrose and threose (aldotetroses) are obtained from glyceraldehyde by adding a fourth carbon. The addition of a fifth carbon to the aldotetroses gives rise to the aldopentoses, and these in turn form the aldohexoses by adding a sixth carbon.
- Details
- Germán Fernández
- CARBOHYDRATES THEORY
- Hits: 19674
Natural ketoses are a family of monosaccharides that contain a ketone group at position 2 of the chain.
- Details
- Germán Fernández
- CARBOHYDRATES THEORY
- Hits: 38941
When studying the optical rotation of natural glyceraldehyde, it was observed that it coincided with the dextrorotatory enantiomer and it was named D-Glyceraldehyde. The levorotatory enantiomer, not present in nature, was named L-glyceraldehyde.
- Details
- Germán Fernández
- CARBOHYDRATES THEORY
- Hits: 32483
The Haworth projection allows drawing the hemiacetal shapes in the plane. In the case of furanoses we have already used this notation in the previous examples, so I will draw an example of a pyranose in that projection.
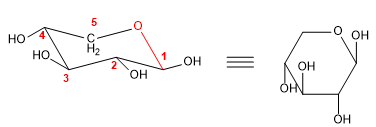
- Details
- Germán Fernández
- CARBOHYDRATES THEORY
- Hits: 28653
The sugars in solution are found mostly in a cyclic form, called a hemiacetal. The hemiacetal is obtained by attacking one of the hydroxyl groups of the chain on the carbonyl. The cycles formed are of five or six members.
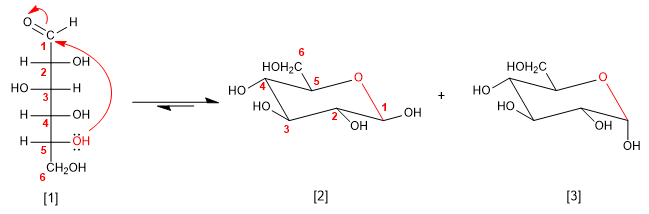
D-Glucose
b -D-Glucopyranose
a -D-Glucopyranose
- Details
- Germán Fernández
- CARBOHYDRATES THEORY
- Hits: 18008
Mutarotation is the interconversion between anomers via the open form. Thus, glucose is found in the aqueous medium as a mixture of alpha and beta anomers and a small amount in open form. Let's look at that balance.
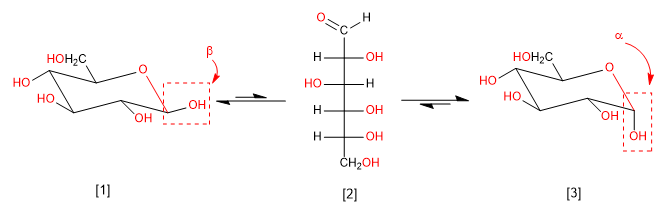
b -D-Glucopyranose (63.6%)
D-Glucose (0.003%)
a -D-Glucopyranose (36.4%)
- Details
- Germán Fernández
- CARBOHYDRATES THEORY
- Hits: 24276
The Kiliani-Fischer synthesis makes it possible to lengthen the monosaccharide chain through the formation and subsequent reduction of cyanohydrins. The big problem of the synthesis is the lack of stereoselectivity.
- Details
- Germán Fernández
- CARBOHYDRATES THEORY
- Hits: 13642
Cu(II) and Ag(I) are capable of selectively oxidizing the carbonyl group of aldoses to carboxylic acid, generating Cu 2 O and elemental Ag precipitates.
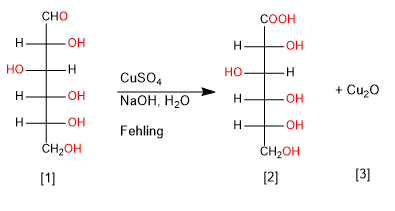
D-Glucose
D-Gluconic Acid
cuprous oxide precipitate









