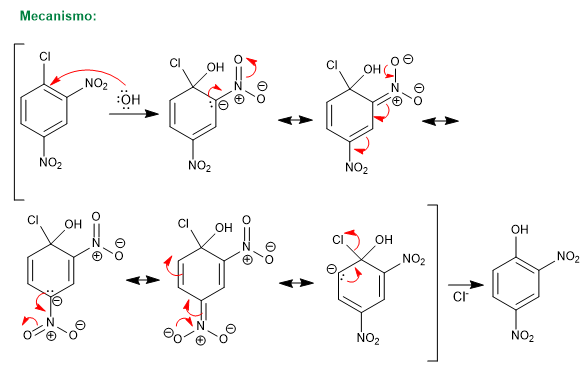
The reaction of 1-chloro-2,4-dinitrobenzene with nucleophiles (hydroxide, ammonia, methoxide, etc.) produces the substitution of chlorine by the corresponding nucleophile. It is called ipso (same place), to indicate that the nucleophile occupies the same position as the starting chlorine.
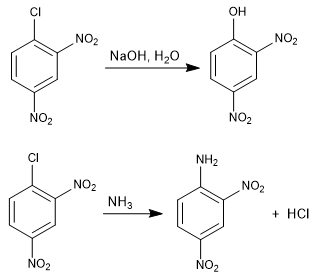
For this reaction to take place, it requires deactivating groups in ortho or para positions with respect to the halogen (groups -NO2 , SO3H). The mission of these substituents is the stabilization of the anionic intermediate that is formed and to remove charge from the ring to enable the attack of the nucleophile.