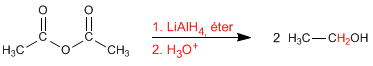ANHYDRIDE THEORY
- Details
- Germán Fernández
- ANHYDRIDE THEORY
- Hits: 144970
Anhydrides come from condensing two molecules of carboxylic acids. The condensation of two molecules of the same acid gives rise to symmetrical anhydrides, which are named by replacing the word acid with anhydride .
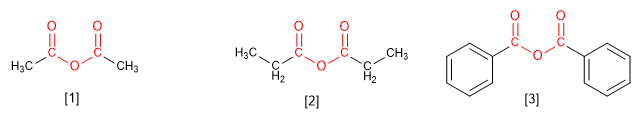
[1] Ethanoic anhydride
[2] Propanoic anhydride
[3] Benzoic anhydride
- Details
- Germán Fernández
- ANHYDRIDE THEORY
- Hits: 68253
- Details
- Germán Fernández
- ANHYDRIDE THEORY
- Hits: 47076
Anhydrides react with alcohols to form esters. The reaction can be carried out without acid catalysis, under slight heating.
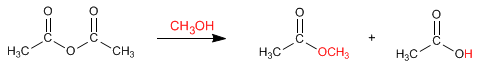
- Details
- Germán Fernández
- ANHYDRIDE THEORY
- Hits: 40967
The anhydrides react with amines and ammonia to form amides. Under the reaction conditions, a carboxylic acid is also obtained.
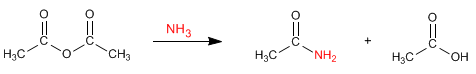
- Details
- Germán Fernández
- ANHYDRIDE THEORY
- Hits: 25818
Lithium aluminum hydride reduces the anhydrides to alcohols. In a first stage the molecule breaks, forming aldehyde and carboxylate which are in turn reduced to alcohols.