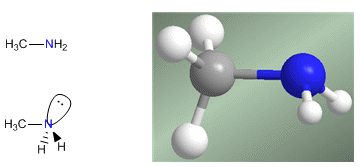
Amines are nitrogenous compounds with a pyramidal structure, similar to ammonia. Nitrogen forms three single bonds through sp 3 -hybridized orbitals. The lone pair occupies the fourth orbital with sp 3 hybridization and is responsible for the basic and nucleophilic behavior of amines.
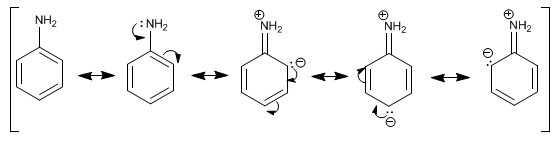
In aromatic amines, such as aniline, the most notable feature is the delocalization of the lone pair on the aromatic ring. This delocalization produces an increase in the electronic density of the phenyl group, increasing the reactivity of aniline in electrophilic substitution reactions.