Amines have lower melting and boiling points than alcohols. Thus, ethylamine boils at 17ºC, while the boiling point of ethanol is 78ºC.
CH 3 CH 2 OH P.b. = 78ºC
CH 3 CH 2 NH 2 P. eb. = 17ºC
The lower electronegativity of nitrogen, compared to that of oxygen, makes the hydrogen bonds formed by amines weaker than those formed by alcohols.
It is also observed that primary amines have higher boiling points than secondary ones and these in turn higher than tertiary ones.
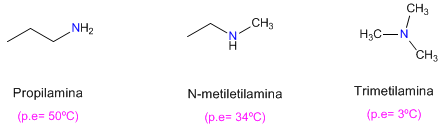
The tertiary amine cannot form hydrogen bonds (it lacks hydrogen on nitrogen), which explains its low boiling point.
In the case of the secondary amine, the steric hindrances due to the chains surrounding the nitrogen hinder the interactions between molecules.
Amines with fewer than seven carbons are soluble in water.









