Allylic systems can act as substrates in nucleophilic substitution reactions. Thus, the reaction of 3-chloro-1-butene with water produces two allylic alcohols.
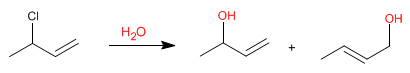
Since water is a bad nucleophile, the substitution mechanism will be unimolecular (S N 1), with the particularity of finding the leaving group (chloro) on an allylic position.
We describe the mechanism below:
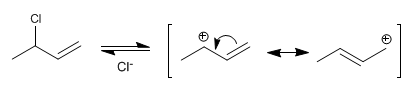
Stage 1 . Formation of the allylic carbocation by loss of the leaving group. The carbocation is stabilized by resonance, the hybrid being formed by two resonant structures.
Stage 2 . Nucleophilic attack of water on the carbocation formed. The carbocation exists in two structures, which when attacked form two different products. The most important structure is the one that locates the charge on the secondary carbon, obtaining the majority product from it (low temperature conditions and short reaction times).
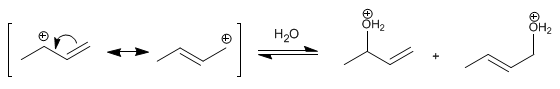
Stage 3 . deprotonation Water loses a proton becoming alcohol.
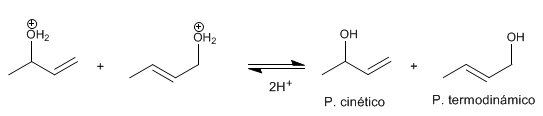
The most stable product (most substituted double bond) is called thermodynamic . The least stable product is called kinetic .