ALKYNE THEORY
- Details
- Germán Fernández
- ALKYNE THEORY
- Hits: 90960
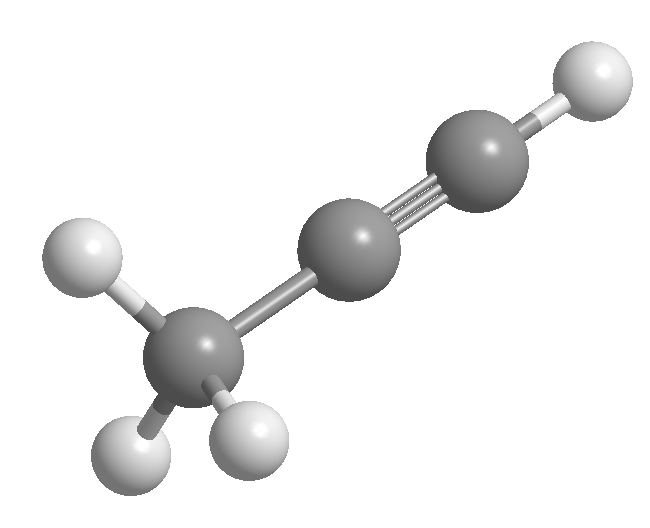
Alkynes are hydrocarbons that contain carbon-carbon triple bonds. The general molecular formula for acyclic alkynes is CnH2n-2 and its degree of unsaturation is two. Acetylene or ethyne is the simplest alkyne, it was discovered by Berthelot in 1862.
- Details
- Germán Fernández
- ALKYNE THEORY
- Hits: 108698
Rule 1. Alkynes respond to the formula $C_nH_{2n-2}$ and are named by replacing the suffix -ane of the alkane with the same number of carbons by -yne.
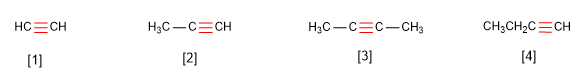
- Details
- Germán Fernández
- ALKYNE THEORY
- Hits: 145125
Alkynes have similar physical properties to alkanes and alkenes. They are poorly soluble in water, have a low density and have low boiling points. However, alkynes are more polar due to the greater attraction that an sp carbon exerts on electrons, compared to an sp 3 or sp 2 carbon.
- Details
- Germán Fernández
- ALKYNE THEORY
- Hits: 78126
The carbon-hydrogen bond of terminal alkynes is polarized and shows a slight tendency to ionize.
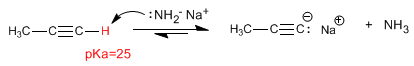
- Details
- Germán Fernández
- ALKYNE THEORY
- Hits: 80118
As we saw in the previous point, terminal alkynes have acidic hydrogens that can be removed by strong bases, forming acetylides (conjugate base of the alkyne). Acetylides are good nucleophiles and give mechanisms of nucleophilic substitution with primary substrates.
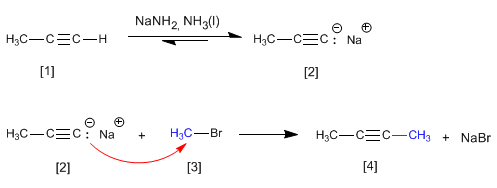
- Details
- Germán Fernández
- ALKYNE THEORY
- Hits: 60225
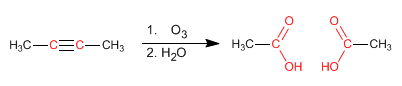
Alkynes can be prepared by double dehydrohalogenation of vicinal or geminal dihaloalkanes.
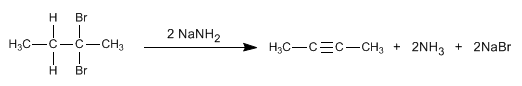
- Details
- Germán Fernández
- ALKYNE THEORY
- Hits: 88412
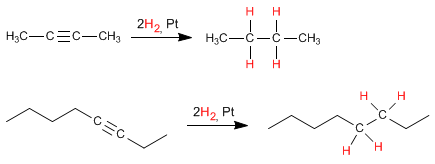
- Details
- Germán Fernández
- ALKYNE THEORY
- Hits: 61516
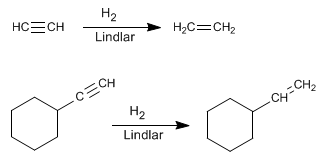
- Details
- Germán Fernández
- ALKYNE THEORY
- Hits: 53480
An alternative to hydrogenation with Lindlar's catalyst is the reduction of alkynes with sodium or lithium in liquid ammonia, the difference lies in the alkene obtained. Thus, hydrogenation with Lindlar's catalyst produces cis alkenes, while hydrogenation with sodium in liquid ammonia generates trans alkenes.
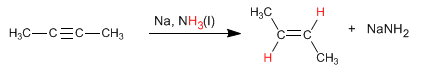
Read more: Hydrogenation of Alkynes with Sodium in Liquid Ammonia
- Details
- Germán Fernández
- ALKYNE THEORY
- Hits: 87412
Alkynes react with aqueous sulfuric acid in the presence of a mercury catalyst to form enols. The enol isomerizes (tautomerizes) rapidly under the reaction conditions to give aldehydes or ketones.
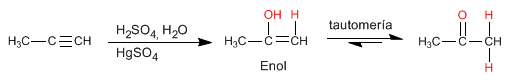
- Details
- Germán Fernández
- ALKYNE THEORY
- Hits: 51346
Hydroboration is the anti-Markovnikov hydration of an alkyne. A hindered borane (dicyclohexylborane or diisoamylborane) is used as a reagent, obtaining an enol that tautomerizes to aldehyde or ketone. Terminal alkynes give rise to aldehydes, internal ones to ketones.
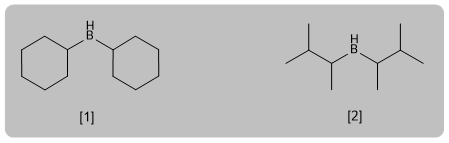
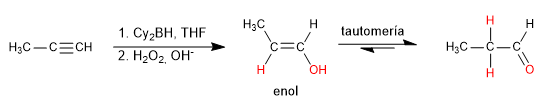
- Details
- Germán Fernández
- ALKYNE THEORY
- Hits: 80275
Similar to alkenes, alkynes add hydrogen halides (HBr, HCl, HI) to the triple bond to form alkenyl halides.
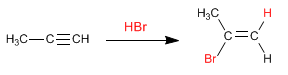
- Details
- Germán Fernández
- ALKYNE THEORY
- Hits: 90944
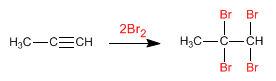
- Details
- Germán Fernández
- ALKYNE THEORY
- Hits: 65650