Alkenes are named by replacing the -ane ending of the corresponding alkane with -ene. The simplest alkenes are ethene and propene, also called ethylene and propylene at the industrial level.
Rule 1.- The longest chain that contains the double bond is chosen as the main chain. Numbering begins at the end that gives the double bond the lowest locant.

[1] 1-Butene
[2] trans-2-Hexene
Rule 2.- The name of the substituents precedes that of the main chain and is accompanied by a locator that indicates its position in the molecule. The molecule is numbered so that the double bond takes the lowest locant.
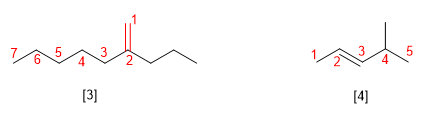
[3] 2-Propylhept-1-ene
[4] trans-4-Methyl-2-pentene
Rule 3.- When there are several substituents, they are arranged alphabetically and are accompanied by their respective locators.
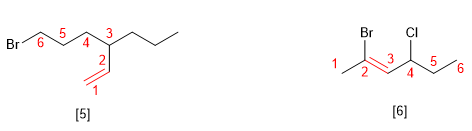
[5] 6-Bromo-3-propylhex-1-ene
[6] (Z)-2-Bromo-4-chlorohex-2-ene
Rule 4.- When the double bond is at the same distance from both ends, it is numbered so that the substituents take the smallest locants.
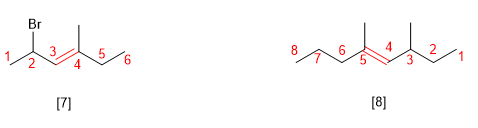
[7] (E)-2-Bromo-4-methylhex-3-ene
[8] (E)-3,5-Dimethyloct-4-ene
Rule 5.- In cyclic compounds it is unnecessary to indicate the position of the double bond, since it is always between positions 1 and 2.

[9] 3,4-Dimethylcyclopentene
[10] 4-Ethyl-3-methylcyclohexene