The following models show the structure, distances, and bond angles of ethene. Each of the carbons in the molecule is $sp^2$ hybridized. Its geometry is flat, with bond angles close to 120º.
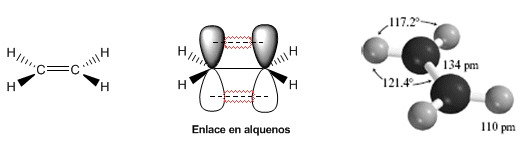
The double bond is formed by a $\sigma$ bond that is obtained by the overlap of the $sp^2$ hybrid orbitals, and a $\pi$ bond formed by the overlap of p orbitals that did not hybridize (pure p orbitals).
The double bond is stronger and shorter than the single one. The energy of the double bond in ethene is 605 KJ/mol compared to 368 KJ/mol for the carbon-carbon single bond in ethane.