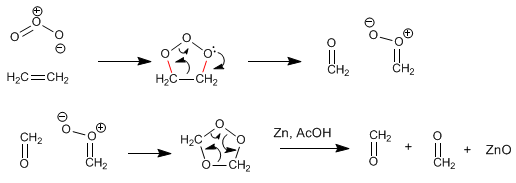
Alkenes react with ozone to form aldehydes, ketones, or mixtures of both after a reduction step.
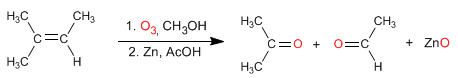
Ozonolysis breaks alkenes, each carbon of the alkene joining an oxygen of ozone, the third oxygen reacts with the reductant.
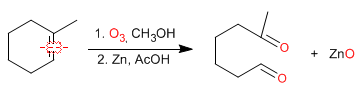
Ozonolysis is an important method for preparing aldehydes and ketones, but it can also be used as an analytical method to determine alkenes. Once the ozonolysis products are known, the structure of the alkene can be determined.
Determine the structure of the alkene that produces cyclohexanone and methanal in an equimolar ratio when broken with ozone.
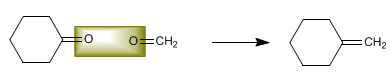
The mechanism of ozonolysis consists of a 1,3-dipolar reaction between ozone (dipole) and an alkene (dipolarophile) to form the molozonide that breaks through the retro-1.3-dipolar generating new dipole and dipolarophile, which through a new 1 ,3-dipolar forms the ozonide. The ozonide breaks down in the reduction stage, leaving the carbonyls free.