Hydrogenation is the addition of hydrogen to a double bond to form alkanes.
Platinum and palladium are the most commonly used catalysts in the hydrogenation of alkenes. Palladium is used in the form of powder adsorbed on carbon (Pd/C). Platinum is used as PtO2 (Adams Catalyst). 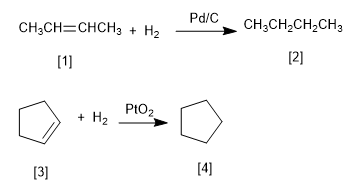
[1] 2-Butene
[2] Butane
[3] Cyclopentene
[4] Cyclopentane
Alkenes are hydrogenated on both sides, producing two enantiomers in equal proportion (racemic mixture).
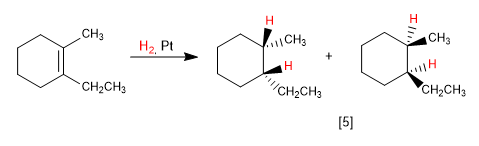
[5] Racemic mixture
When the alkene has one face more hindered than the other, diastereoisomers are obtained in different proportions.
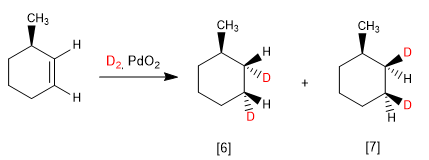
[6] Major product of hydrogenation. The underside of the alkene is less hindered.
[7] Minority product. Hydrogenation occurs on the methyl side, which prevents the approach of the reagent
In bicycles the most favorable face is the one on the side of the bridge:
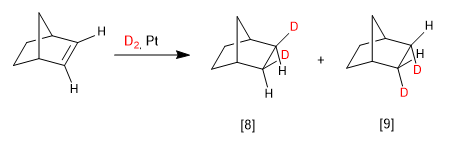
[8] Major product. The bridge of the bicycle is the chain that exerts the least impediment.
[9] Minority product.