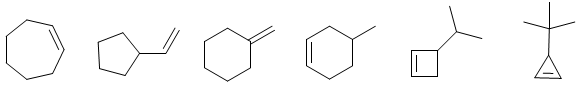
Draw the compound of molecular formula C7H13 Br that each alkene shown as unique elimination product E2 forms.
SOLUTION:
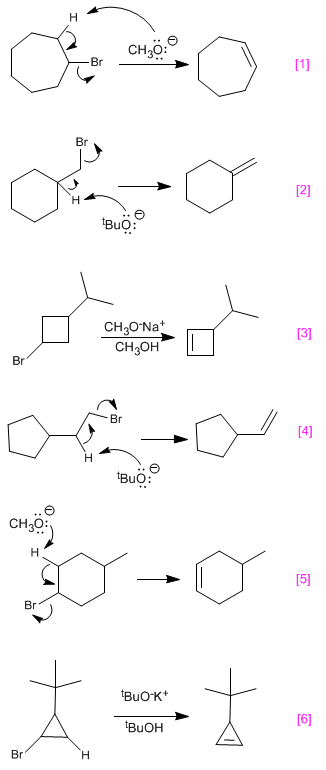
To obtain the haloalkane that generates the alkene by elimination, bromine must be placed on one carbon of the double bond and hydrogen on the other. In the example both carbons are the same and the order in which both groups are placed does not matter.
If the bromine must be placed on the end carbon so that only one b carbon exists and elimination regioselectively generates the desired alkene. The use of tert-butoxide is due to the fact that the primary substrates give S N 2 with strong bases, but eliminate with hindered bases.
In bromine is placed in front of isopropyl, so that the two b carbons are equivalent and only the desired alkene is obtained.
In the desired alkene is achieved by placing the bromine at the end of the chain and using hindered base to avoid SN2
In a symmetrical position for the bromine is also sought, thus achieving the formation of a single alkene.