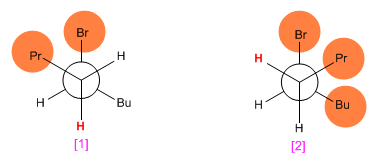
In the elimination reaction of 5-bromononane with potassium ethoxide in ethanol, draw Newmann projections showing the conformation leading to cis-4-nonene and trans-4-nonene, respectively. Indicate the hydrogen that is lost in each case and suggest a mechanism that explains the observed stereoselectivity.
Solution
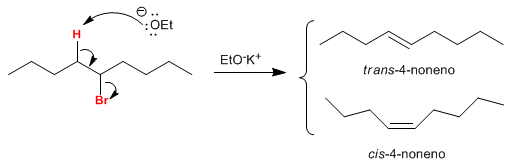
On carbon b of the haloalkane there are two hydrogens that, when placed anti with respect to the leaving group, generate the two isomers. The trans isomer is obtained in greater quantity, since the transition state of the reaction that forms it is of lower energy.
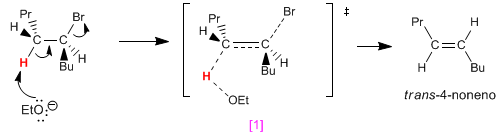
Rotating the carbon on the left 120º, we place the black hydrogen anti with respect to the bromine. Removal of this hydrogen forms the cis alkene.
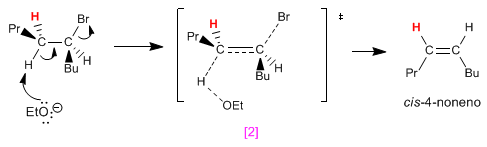
Making Newmann projections of both conformations, it is observed that the [1] has fewer repulsions (propyl and butyl to opposite sides) than [2] . This causes the trans isomer to be obtained in greater quantity than the cis (the reaction that forms the trans product is faster because it has lower activation energy).