menthyl chloride and that of neomenthyl They have the structures shown below.
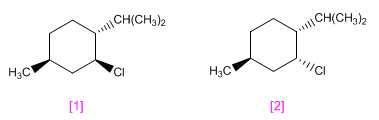
One of these stereoisomers undergoes elimination by treatment with sodium ethoxide in ethanol much more readily than the other. Which will react faster? Because? Indicate the resulting product.
SOLUTION:
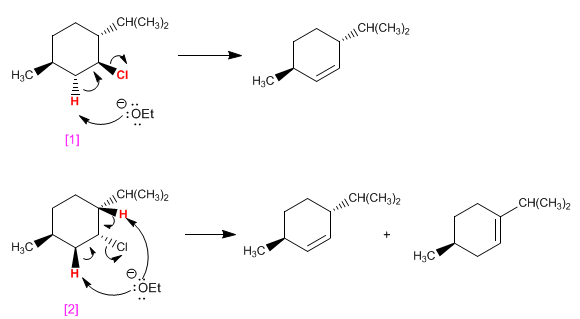
the molecule [1] it has a single hydrogen ANTI with respect to chlorine and eliminates giving a single alkene. the molecule [2] has two ANTI hydrogens, forming two products in bimolecular elimination.
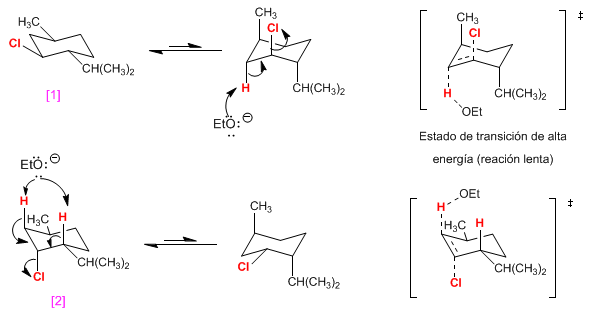
the molecule [1] It is eliminated in a high energy conformation -with all the groups in an axial position- and the reaction will be slow because it has a high activation energy.
the molecule [2] it has only the chlorine in axial and will give rise to a transition state of lower energy. faster reaction