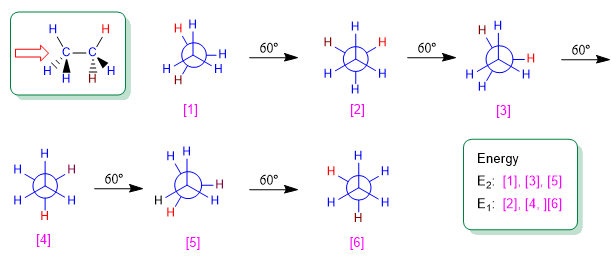
Ethane is the simplest alkane that can exist in different conformations. Of the infinite conformations that can be obtained by turning the carbon-carbon bond, the alternated and eclipsed conformations stand out for their importance.
Alternate conformation of ethane
As can be seen in the figure, the alternate conformation has the hydrogens of the first carbon located between the hydrogens of the second carbon, there are no opposite hydrogens. 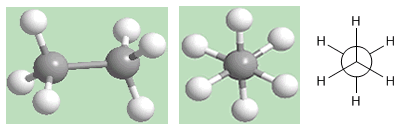
In this conformation, the carbon-hydrogen bonds of both carbons are far apart, forming 60º angles. The staggered conformation is the most stable of the ethane conformations. That is, the one with the lowest energy.
Eclipsed conformation of ethane
The second conformation is called eclipsed. In it, the carbon-hydrogen bonds of both carbons face each other (eclipsed). The electronic fields of the opposing hydrogens suffer important repulsions (eclipsements) that destabilize the conformation. The eclipsed conformation of ethane is the one with the highest energy. That is, the most unstable. 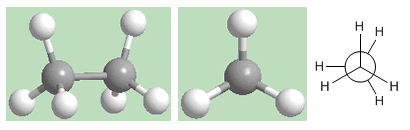
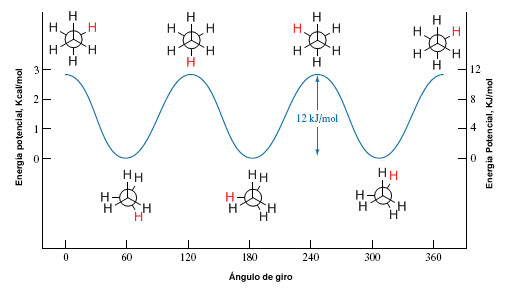
Rotation about a carbon-carbon single bond is not completely free, due to the energy differences between the staggered and eclipsed conformations. For the rotation to take place it is necessary to overcome an energy barrier that is given by the energy of the eclipsed conformation (activation energy). In ethane this energy barrier is small, about 2.9 Kcal/mol (12 KJ/mol), and allows the conformers to interconvert at high speed.
potential energy diagram
A potential energy diagram allows us to understand how the potential energy of the system changes during rotation. These diagrams represent the potential energy of the molecule against the angle rotated.