 Single bonds between atoms have cylindrical symmetry and allow rotation of the groups of atoms attached to them. The different spatial arrangements that the atoms adopt as a result of rotation around the bond are called conformations. A particular conformation of many possible ones is called a conformer.
Single bonds between atoms have cylindrical symmetry and allow rotation of the groups of atoms attached to them. The different spatial arrangements that the atoms adopt as a result of rotation around the bond are called conformations. A particular conformation of many possible ones is called a conformer.
The rotation of the carbon-carbon bond in ethane gives rise to two limit conformations -the staggered conformation (with alternate hydrogens) and the eclipsed conformation (with opposite hydrogens). The transition from the alternate to the eclipsed conformation or vice versa is performed per 60º turn Observe that in a 360º turn there are infinitely many possible conformations. 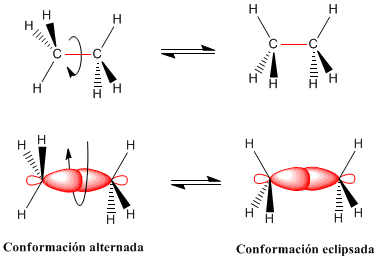
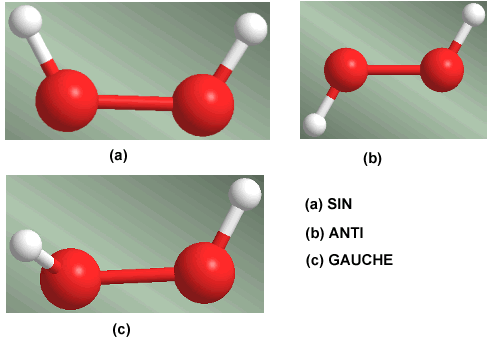
Rotation around the oxygen-oxygen single bond in the hydrogen peroxide molecule generates three conformations of special importance. The conformation that has the hydrogens facing each other is called SIN. When the hydrogens are located on opposite sides, it is called an ANTI conformer. The conformation that leaves the hydrogens at 60º is called Gauche.